
Affected fish often have other concurrent or secondary infections. Nocardiosis begins as a silent infection, developing undetected for months in fry or juvenile fish. The duration of the infection is a long-term (chronic) phenomenon. Nocardia bacteria multiply slowly within fish tissues before any visual symptoms arise, and certainly before lethargy and death rates increase.
The typical outcomes within affected fish populations are:
- poorly performing yearling and pre-harvest fish
- elevated feed conversion rates
- emaciation
- rising mortality rates near the end of the summer
Causative Agent
Many Nocardia species are found in the terrestrial and marine environments, but Nocardia seriolae (previously, N. kampachi) is considered the most likely pathogen of Seriola fish. The bacteria do not stimulate a septicaemic reaction or an acute immune response. Rather, Nocardia is thought to progressively invade (and perhaps dwell and multiply inside) various types of fish host cells, including white blood cells. Relatively limited information about the microbiology, chemistry and patho-physiology related to Nocardia is published. This may be due to the inherent problems of researching slow-growing microorganisms.
Transmission and Epidemiology
The initial exposure of fry to Nocardia is the likely result of the fry consuming uncooked fish tissues (live, raw or frozen) or by the horizontal transmission of Nocardia from sick fish. Amberjack and yellowtail juveniles fed raw fish or moist pellets are likely the first to be infected, so the use of raw, low quality, trash fish should be avoided when rearing fish of any type. The infection develops silently as the bacteria multiply slowly over months within major organs, such as the spleen, kidney and liver.
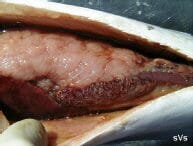 Spleen with hundreds of 1- to 2-mm white-yellow spots. |
In marine finfish culture, nocardial infections appear to progress more quickly during the summer months when water temperatures reach 24C or more, but the mortality due to Nocardia is more commonly experienced in the autumn and early winter months, perhaps as the fish becomes overwhelmed and its immune system wanes.
Clinical signs and Gross Pathology
 A classic nocardial lesion: whitish-yellow irregularly-shaped masses at the base of the gill filaments. |
 A coalescing cluster of nocardial skin abscesses. These dry abscesses protrude individually or in groups, each containing massive numbers of Nocardia bacteria. Many burst leaving a dry yellow skin ulcer. |
The white-yellow granulomata are usually 1-2 mm in size. The spots are most obvious in the spleen, kidney and liver but can be found in any tissue. Fish that mount a significant immune reaction to the disease eventually heal somewhat and exhibit hard black spots (melano-macrophage accumulations) in place of the white spots in the liver and adipose tissues. Brown-black crusty plaques often develop on the dorsal inner surface of the swim bladder.
Microbiology
The bacterium is thread-like, beaded and branching. It is variable-staining when using Grams stain and the bacteria are acid-fast positive (pink). The culture and isolation of Nocardia is relatively easy, yet somewhat tedious. Several types of agar and broth media will support nocardial growth, but these media also support the growth of other faster-growing species of bacteria. The use of selective antibiotic agar-tube media, such as Lowenstein-Jensen, is most efficient to isolate Nocardia directly. The incubation time may range from 4-10 days depending on incubation temperatures of 25C to 35C. The colonies appear dry and stacked. The results of in vitro antibiotic sensitivity testing tend to be ambiguous and mis-leading. In general, Nocardia appears to be inherently resistant to most commercially available antibiotics when challenged in vitro and in vivo.
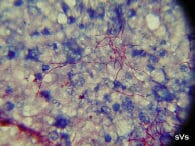 A spleen imprint or stamp (x1000, acid-fast stain) showing bright pink, thread-like branching and beaded Nocardia seriolae. |
Diagnosis and Primary on-site tests
A thorough visual examination of the fish is always the best way to begin an assessment. Feel the skin and body wall for lumps and ulcers. Upon cutting through the firm skin nodules, one will find a dry, grey-yellow, inspissated abscess. Lift the operculum to look for pale gills and irregular whitish lumps at the base of the filaments.
Gill, kidney or spleen imprints or stamps are easily collected (in duplicate), dried and stained using Grams or an acid-fast stain. Five-mm sections of the same tissues are helpful for a histological diagnosis when preserved in 10% buffered formalin.
Management and Control
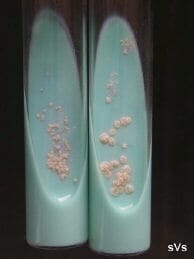 Colonies of Nocardia seriolae isolated on Lowenstein-Jensen selective (antibiotic) agar. |
The best prevention and control of this disease would be through vaccination; however, Nocardia vaccine development remains experimental. To date, I am unaware of the development of a commercially available, efficacious antigen-adjuvant combination that will prevent nocardial infections in fish. Therefore, the early detection of silent infections amongst juvenile live fish is the goal. However, the efficacy and practicality of detecting sub-clinical nocardiosis remains questionable in that the surveillance for infection may involve expensive experimental tests, such as: mucus testing by polymerase chain reaction (PCR) and antibody serology.
The use of drugs to control Nocardia is controversial. Environmentally, consumers are not in favour of drug treatments. From a fish production viewpoint, it is very difficult to ensure that fish will consume sufficient volumes of
medicated feed to achieve a therapeutic daily dose. In making the attempt, the fish may reduce their daily food intake and slow their weight gain, thus creating another cost to the farmer. Overall, the cost-effectiveness of antibiotic therapies to control nocardiosis in finfish is debatable.
Antibiotic doses need to be high and the duration of treatment must be extended to the point that the use of antibiotics is largely impractical. That said, it is speculated that the application of two prophylactic treatments applied to asymptomatic juvenile fish may be useful. Using specific antibiotics that can penetrate fish cells (when in high serum concentration) for an extended period of time (i.e., 10 - 14 days) may interfere with in vivo nocardial development.
Control and prevention through husbandry and good management practices is the best approach for nocardial infections. Avoid the use of uncooked fish feeds (live, raw or frozen) when rearing fish of any age or type. Feed only dry cooked feed. Reduce the shellfish fouling (i.e., barnacles) on floats and ropes near your finfish cages whenever possible. Disinfect hands and marine equipment, practice strict diving hygiene between pens, farm sites and rearing areas, and minimize fish stress as much as possible.
Key References
Sheppard, M.E. (2004, 2005): A photographic guide to diseases of yellowtail (Seriola) fish. 64 pages. ISBN 0-920225-14-4, 0-920225-15-2.
Miyoshi, Y. and S. Suzuki (2003): A PCR method to detect Nocardia seriolae in fish samples. Fish Pathol., 38, 93-97.
Itano, T. and H. Kawakami (2002): Drug susceptibility of recent isolates of Nocardia seriolae from cultured fish. Fish Pathol., 37, 152-153. (in Japanese with English abstract)
Fukuda, Y. (2001): Prefectural disease report PCR and in vitro comparisons of nocardiosis, mycobacteriosis, odan. 11, 131-139. (in Japanese)
Sako, H. (1988): Survival of fish pathogenic bacteria in seawater. Bull. Natl. Res. Inst. Aquaculture, 13, 45-53. (in Japanese with English abstract)
Kusuda, R. and A. Nakagawa (1978): Nocardial infection of cultured yellowtail. Fish Pathol., 13, 25-31. (in Japanese with English abstract)
Originally Published May 2005

