
SRS was first reported, from Chile, in 1989, but (Pisci)rickettsia-like organisms (RLO) are now frequently associated with disease syndromes in both salmonid and non-salmonid fish from both fresh and saltwater worldwide. During 1989, this disease was considered to be the cause of death of an estimated 1.5 million Coho salmon, many near market-size. A year later, the disease was also found to occur in Atlantic salmon and up to 90% mortality was seen on some farms. Outbreaks of SRS in other countries have not reached the levels of the Chilean outbreaks. For example, variable and inconsistent mortality of 0.6 - 15% has been reported in Canada and Norway.
Causative agent
SRS is caused by the Gram-negative bacterium, Piscirickettsia salmonis. This was the first "rickettsia-like" bacterium to be recognized as a pathogen of fish. P. salmonis is a non-motile, obligate intracellular bacterium, pleomorphic but predominately coccoid, and 0.5-1.5 m in diameter. It is currently placed in the class Gammaproteobacteria; order Thiotrichales; and family Piscirickettsiacaea, and has a closer relationship to, e.g., Legionella and Coxiella, than to members of the genera Rickettsia. P. salmonis replicates within membrane-bound cytoplasmic vacuoles in selected fish cell lines and in the cells of tissues throughout infected fish.
P. salmonis is the first of the RLO of fish to be fully characterized. Since its recognition, the impact of RLO in fish has become increasingly apparent. Growing awareness of the emergence of these intracellular organisms has led to the discovery of rickettsial diseases among diverse species of fish from different geographic locations and aquatic environments. The source, reservoir, and mode of transmission of many of these agents, as well as consistently effective methods of disease prevention and control, remain to be established.
Host range, geographic distribution SRS disease in Chile typically occurs in marine waters during the on-growing process from smolt to harvest. It has also been isolated from freshwater cages of Coho salmon and trout. The disease was originally predominant in Coho salmon (Oncorhynchus kisutch) but is now recognized to cause serious losses in all farmed salmonid fish species including Atlantic salmon (Salmo salar), rainbow (steelhead) trout (O. mykiss), Chinook salmon (O. tshawytscha), pink salmon (O. gorbuscha) and masu salmon (O. masou). Piscirickettsia sp. are commonly found in fish worldwide (e.g., Chile, Canada, Ireland, Scotland and Norway) but are of major economic importance only in Chile to date. The distribution of P. salmonis and RLO is therefore wide spread. Several reports describing RLO infections in non-salmonid finfish exist. For example, about 10 years ago, a RLO was identified as the causative agent of an outbreak with mass mortality among pond-reared tilapia in Taiwan. Also, RLO-related mortalities in juvenile European sea bass at 12 15 C in sea cages have been reported along the French Mediterranean coast.
Transmission and epidemiology
At present there are few reports of P. salmonis coming from wild salmonids, although it is likely that the bacterium is present in naturally occurring populations of marine fish. Horizontal transmission has been reported in marine-farmed salmon 2 weeks after the introduction of pathogen-free fish into infected sites. The extended extracellular survival time of this organism in salt water (several weeks at 5-20 C) may be of sufficient duration to permit horizontal transmission without a vector. Experimentally it is documented that the bacterium can enter through the intact skin and gills although the mode of entry is still not clear.
The possibility of vertical transmission of P. salmonis now looks more and more likely due to recent research in Chile. Apparently there is an adhesion complex that allows the pathogen to enter the salmon egg. There is even a suggestion that this complex may be involved in fish to fish transmission.
Currently, no alternative host has been identified and the source, reservoir and means of transmission of P. salmonis remain important areas of research.
The course of the clinical disease is typically chronic to subacute in nature with mortalities typically developing 10 - 12 weeks after the transfer of fish to seawater and lasting approximately 10 weeks before they diminish. Virtually all stocks become infected and usually experience more then one clinical episode, typically in the spring and autumn seasons.
Clinical signs and gross pathology
Histopathology
Histological changes have been classified into the broad category of necrosis and inflammation. Inflammatory cells, fibrosis, a generalized coagulative necrosis, tubular degeneration and necrosis of the endothelium infiltrate the liver, spleen, intestine and haematopoietic cells of the kidney. Moribund fish are anaemic and haematocrit is often 20% to 50% of normal. The rickettsial organism infects a variety of cells, including circulating macrophages, in which they can replicate and cause cell lyses. It also enters brain tissue, thus affecting swimming ability. The mechanisms by which P. salmonis can enter target cells, avoid intracellular killing and survive inside the host are unclear.
Diagnosis
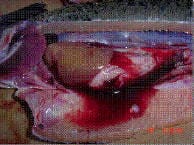 SRS pathology in salmon. Note the severe inflammation and multifocal necrosis in liver and spleen. Haemorrhagic ascites are also observed. |
An initial diagnosis of piscirickettsiosis can be made from gross lesions and is supported by the examination of tissue sections. Confirmation of the diagnosis requires isolation and/or serological identification of the causative organism. Kidney tissue from affected fish is aseptically removed, homogenized and inoculated on a cell monolayer with an antibiotic-free growth media. P. salmonis has been cultured in many (mostly salmonid) fish cell lines maintained in buffered Eagles minimum essential medium (MEM) supplemented with 10% foetal bovine serum.
Optimal in vitro growth occurs at 15 18 °C but is retarded above 20 °C and below 10 °C. [Due in part to this thermal range, there is no indication that P. salmonis or other RLO of fish cause disease in humans or other mammals.] Typically, P. salmonis isolation and growth is determined by the gradual appearance of a typical cytopathic effect (CPE) in cell monolayers. The first signs of a CPE consist of the formation of cell clusters about 10 days post-inoculation. The infected cells in the clusters typically round up and develop one or more large vacuoles within the cytoplasm. Inoculated cell cultures should be observed for up to 28 days before they are considered negative.
An indirect fluorescent antibody technique (IFAT) and immunohistochemistry have been developed as alternative procedures to detect P. salmonis. These latter techniques are faster and more specific than histochemical staining. However, they require additional specialized equipment and are more expensive. The detection of P salmonis in cultivated salmonids via a nested PCR using universal primer is coming on stream and will be important for diagnosis of this disease.
Management and Prevention: Chemotherapy
In vitro, P. salmonis is sensitive to a variety of antibiotics including streptomycin, gentamicin, erythromycin, chloramphenicol and oxytetracycline, but shows resistance to penicillin, penicillin G and spectinomycin. However, the use of medicated feed to control intracellular pathogens, including P. salmonis, has been largely unsuccessful, possibly because antibiotic levels may not reach sufficient concentrations within the host cells in vivo. However, injection of broodstock with antibiotics before leaving seawater in order to control the typical summer SRS outbreak is common.
Vaccine development
Although commercial vaccines against P. salmonis are very recently available, there is little published information or field experience on their efficacy or economic value. However, several institutes and pharmaceutical companies, including Intervet, have active research programmes directed towards developing efficacious vaccines.
Management
Outbreaks frequently occur after smolt transfer to seawater, but good management practices do help. Such approaches include the early removal of mortalities and clinically diseased fish, with appropriate sanitary disposal of blood from harvested fish, reducing fish stocking density and providing periods of site fallowing. Other strategic measures include routine screening of broodstock, rejection of eggs from positive fish and individual incubation of egg batches. Further information regarding horizontal and vertical transmission, pathogenesis, intracellular survival and immunogenesis is needed to support future control strategies. In addition, information on the geographic location and species distribution of P. salmonis among isolates and stocks of fish will be helpful in developing management and control strategies in the future.
Key References
Bruno DW, Woo PTK. Sporadic, emerging diseases and disorders. In: Diseases and disorders of finfish in cage culture. Woo PTK, Bruno DW, Lim LHS (eds.) CAB International. pp. 305-343, 2002.
Fryer JL, Mauel MJ. The Rickettsia: an emerging group of pathogens in fish. In: Emerging Infectious Diseases, Vol. 3, No. 2 April--June 1997 pp. 137-144.
Kent ML, Poppe TT. Infectious diseases of coldwater fish in marine and brackish water. In: Diseases and disorders of finfish in cage culture. Woo PTK, Bruno DW, Lim LHS (eds.) CAB International. pp. 61-105, 2002.
September 2006


