Introduction
Aquaflor (florfenicol) is a fast-acting,
palatable, broad-spectrum antibiotic
provided as a feed premix. It is proven to
be an effective and safe antibiotic for use
in fish. To maintain the efficacy of this
valuable antibiotic and obtain maximal
benefit from feed medicated with
Aquaflor, producers and diagnosticians
must understand the rationale behind
the label indications and they must
work cooperatively.
Aquaflor is approved for use in finfish in
over 20 countries. Indications differ from
country to country but include control
of mortality due to diseases associated
with the warmwater bacterial pathogens
Edwardsiella ictaluri, Streptococcus iniae,
Streptococcus agalactiae, Flavobacterium
columnare, Francisella asiatica and
Aeromonas hydrophila.
In the US, the approval of Aquaflor for use
in fish involved many experimental efficacy
and safety studies required by the United
States Food and Drug Administration, a
US regulatory body. In the US, Aquaflor is
currently approved for control of mortality
due to enteric septicemia of catfish,
furunculosis and coldwater disease in
salmonids. Aquaflor-CA1 (florfenicol) is conditionally approved for control of
mortality due to columnaris disease
in catfish.
The objective of this paper is to explain the
best practices for incorporating Aquaflor
into overall disease management programs
for warmwater fish production.
Efficacy Studies
In vivo and in vitro efficacy studies with
Aquaflor have been conducted.
In vitro efficacy studies: The sensitivity
of bacterial pathogens to florfenicol (FFC)
has been assessed by determining the
minimal inhibitory concentrations (MIC)
of FFC for bacteria from both experimental
studies and from diagnostic specimens
obtained from disease outbreaks on farms.
This laboratory assay quickly determines
the susceptibility of bacteria to drug.
MIC values for warmwater fish pathogens
of interest are presented in Table 1. The
values indicate that a wide variety of
warmwater bacterial pathogens are
susceptible to FFC. MIC values correlated
with data from pharmacokinetic studies
can be used to predict and validate the
clinical performance of Aquaflor on farms.
Minimal Inhibitory Concentration (MIC) of Florfenicol in Bacteria Cultured from Catfish and Tilapia. The Values Indicate that the Bacteria are Susceptible to the Antibiotic.
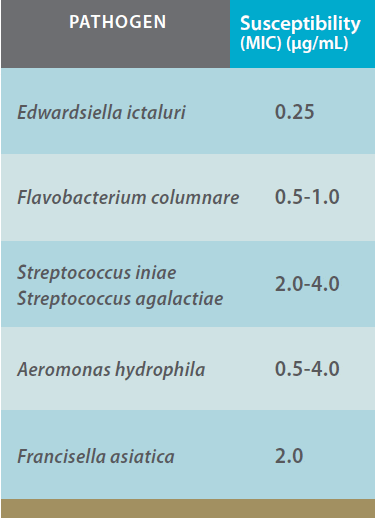
In vivo studies: Experimental Aquaflor efficacy studies in warmwater fish were conducted at a dose rate of 10 mg FFC/kg bodyweight against the pathogens E. ictaluri and F. columnare in catfish (Ictalurus punctatus) fingerlings. Aquaflor was highly efficacious (p < 0.001) when compared to controls (Figures 1 and 2).
Efficacy of Aquaflor Demonstrated by Reduced Mortality in Channel Catfish Challenged with E. Ictaluri, then fed Feed Medicated with Aquaflor at 10 mg FFC/kg of Fish Bodyweight for 10 days. The Feeding Regimens were Initiated 24 Hours After Exposure to E. Ictaluri.
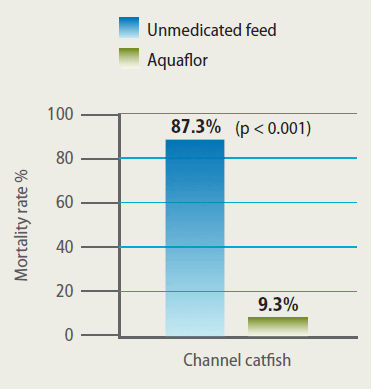
Efficacy of Aquaflor Demonstrated by Reduced Mortality in Channel Catfish Challenged with F. Columnare, then fed Feed Medicated with Aquaflor at 10 mg FFC/kg of Fish Bodyweight for 10 Days. The Feeding Regimens were Initiated 24 Hours After Exposure to F. Columnare.
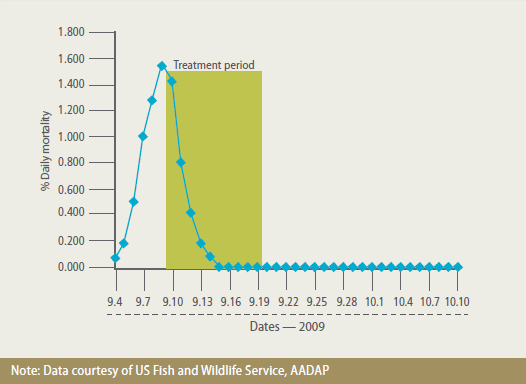
In 2009, high mortality in market-size catfish (~2 lbs or 0.907 g) associated with A. hydrophila occurred on several farms in Alabama, USA. Aquaflor was used to contain the outbreak. There were no untreated controls in these studies for both animal welfare and economic reasons. Nonetheless, comparison of mortality before and after treatment with feed medicated with Aquaflor showed a dramatic decrease and, in some cases, complete cessation of mortality (Figure 3).
Mortality Among Fish Treated at one of Several Farms in Alabama with an A. Hydrophila Outbreak. Over 200,000 Channel Catfish Received 10 mg FFC/kg Fish/Day for 10 Days.
Pharmacokinetic and Residue Depletion Studies
Pharmacokinetic studies were conducted
to determine the disposition of FFC in
warmwater fish (reared in freshwater) and
to confirm the dose rates determined by
efficacy studies. After oral dosing of catfish
at 10 mg/kg (Figure 4) and tilapia at 15
mg/kg, a high concentration of FFC was
absorbed quickly from the intestine, rapidly disseminated throughout the body and
maintained at a steady concentration
during the 10-day dosing period.
Tissue concentrations of FFC in catfish
and tilapia during the 10-day treatment
under the specified experimental
conditions were greater than the MIC
values of five pathogenic bacteria studied
(Table 1). This tissue concentration was
considered sufficient to effectively
combat the pathogenic bacteria.
In a single oral-dose study conducted
with catfish, the mean concentration of
Aquaflor in plasma declined below
1 ?g/mL by 36 hours; this concentration
is below the highest MIC efficacy value
for most catfish pathogens (Table 1).
Therefore, it is critical to continue daily
dosing (every 24 hours) for 10 days to
achieve and maintain an efficacious
dose at the rate necessary to combat
bacterial infections.
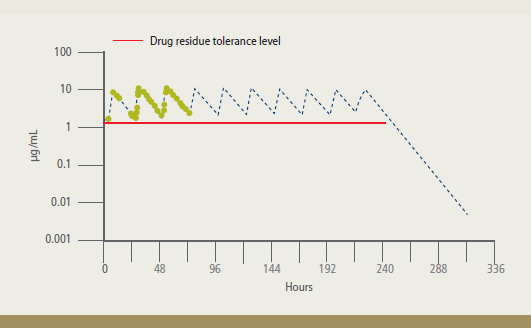
Residue depletion studies were conducted
to determine the calculated withdrawal time, which is the time between the last
dose of FFC and the time when the drug
residue levels fall below the 1 ?g/g
tolerance. The results support a withdrawal
time of 12 days for warmwater fish following
treatment with Aquaflor administered
according to label directions.
Best Treatment Practices
Feed medicated with Aquaflor should
be used in conjunction with proper husbandry,
available vaccines and genetically
improved fish. Good fish husbandry
includes environmental management,
attention to stocking density, biosecurity
and good recordkeeping.
The ideal aquaculture environment is
maintained at optimal water temperatures,
dissolved oxygen and chemistries for each
warmwater species. Although emphasis
is placed on increased stocking rates to
maximize production, fish should be
stocked near, but not in excess of, the
maximal capacity to avoid poor water
quality, suboptimal dissolved oxygen
concentrations and traumatic injury.
Any of these factors could stress and
predispose fish to disease.
Biosecurity measures such as footbaths
and cleaning, disinfecting and rinsing
equipment will help prevent the spread
of infectious agents on the farm. Diseases
can be transmitted by humans, predators and scavengers, equipment and water.
Although predators and scavengers are
difficult to deal with, the control of other
vectors is manageable. Newly introduced
fish should be quarantined (and treated
if necessary) for 3 to 6 weeks before
introduction into the growout facilities on
farms that share common water systems.
Proper farm management requires
managers to maintain fish health records
that help enable recognition of diseases
and their proactive management.
Important farm records include the
seasonality of outbreaks, fish eating
patterns and behavior. For example, if
fish become anorexic during summer
when water temperature ranges from
24 C to 28 C (75 F to 82 F), the problem
pathogen is more likely to be S. agalactiae
rather than S. iniae.
Producers should submit live, sick fish
to a diagnostic laboratory for examination
of lesions and culture of the suspected
pathogenic bacterium. Dead fish often
have been contaminated with additional
bacteria that are not the source of a disease
outbreak, which is why live, sick fish
should be examined and lesions cultured;
the results must be carefully interpreted to
avoid an incorrect diagnosis.
It is advantageous for producers to
stock robust fish strains bred for fast
growout and high survival rates. Genetically improved stock fed optimal
diets yield high quality alevins resulting
in larger, more disease-resistant juveniles
that perform well in the field.
Prevention of disease is increasingly
becoming a reality due to the availability
of warmwater fish vaccines, which vary
from country to country. Vaccines
against E. ictaluri and F. columnare
(AquaVac-ESC and AquaVac-COL) in
catfish are commercially available in the
US; AquaVac Strep Sa is the first-ever
intraperitoneal vaccine for tilapia and
protects against S. agalactiae Biotype II,
an important cause of streptococcosis. However, vaccinated fish can still succumb
to pathogens if they are stressed and if
husbandry is less than optimal.
FFC Plasma Concentration Versus Time in Channel Catfish (Ictalurus Punctatus) at a Mean Water Temperature of 25.4 C (77.7 F) After Medication with Aquaflor at a Dose Rate of 10 mg FFC/kg Bodyweight. FFC was Rapidly Absorbed and Attained Effective Plasma Concentrations Above the MIC of most Catfish Pathogens.
Use Aquaflor at the First Sign of Disease, According to Label Directions
Feed medicated with Aquaflor should be
administered as soon as bacterial disease
is recognized in fish. A delay of just a few
days after the onset of disease signs can
mean the difference between successful
therapy and treatment failure.
Important bacterial pathogens of freshwater
tilapia include S. iniae, S. agalactiae,
A. hydrophila, F. asiatica, F. noatunensis
and F. columnare. Fish infected with
the first four of these pathogens will
quickly lose their appetites, which
decreases medicated feed intake.
Experimental studies of F. columnareinfected
catfish demonstrate that the
bacterial infection does not cause
anorexia in catfish.8 However, anecdotal
reports indicate that appetite is affected
in columnaris disease under field
conditions when the mouth becomes
extremely necrotic. As stated previously,
FFC tissue concentration must exceed
its MIC value for the bacteria during the
treatment period. Fish that are not eating
or only eating small quantities will be
inadequately medicated.
Lethargic fish will likely have lesions that
are more indicative of a given disease than
are clinical signs. Streptococcosis in tilapia
is most frequently caused by S. agalactiae
and S. iniae. External lesions include
exophthalmia bulging eyes with corneal opacity (Figure 5),
darkening of the skin, necrosis of gills
and hemorrhage of the skin, opercula,
vent and muscles. Internal lesions include
fluid in the coelomic cavity, hemorrhage
and enlargement of internal organs as
well as inflammation in joints and
the heart.
Corneal Opacity with Exophthalmia (Bulging Eyes) seen in Tilapia with Streptococcosis

A. hydrophila in tilapia is characterized by
external lesions of hemorrhage, ulceration
and necrosis of the skin, base of the fins
and occasionally the muscle. Exophthalmia
is occasionally reported with this disease.
Internal lesions include bloody fluid in
the coelomic cavity and hemorrhage in
the organs.
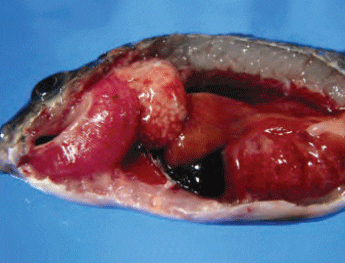
Francisellosis in tilapia is caused by
F. asiatica and F. noatunensis. Typical
lesions of Fransicella spp. infection include
exophthalmia, whitish nodules (which
represent areas of necrosis) in the gills, skin ulcerations (from secondary infections)
and enlarged internal organs, especially
of the spleen and kidney, with whitish
necrotic nodules (Figure 6).
Columnaris disease caused by F. columnare
in tilapia is characterized by external
lesions of ulceration and necrosis of the
gills, mouth, skin, muscle and fins, giving a
ragged appearance of the fin rays caused
by sloughing of the epithelium.
Because Aquaflor requires a prescription
in many markets, such as the US and
Brazil, a veterinarian or other fish health
specialist should examine affected fish
and determine that they are sick based
on the signs of disease, lesions or the
results of diagnostic tests such as bacterial
culture.18 If the fish have a bacterial
disease that is treatable with Aquaflor,
the veterinarian will issue a medicatedfeed
order. Dosing should be followed
according to label directions, using the
effective approved dose rate for 10 days
duration. A dose rate of 10 mg/kg bodyweight
in catfish is efficacious against
E. ictaluri and F. columnare; in tilapia alevins
and juveniles, this dosage has been shown
to be efficacious against F. columnare,
A. hydrophila and S. agalactiae.
Medicated feed should be purchased
from a feed mill that follows Good
Manufacturing Practices to ensure
quality, purity and the indicated label
concentration. For practical reasons, the Aquaflor feeding rate (calculated
as a percentage of bodyweight) for
medicated feed may be predetermined
by commercial feed mills on the basis
of local feeding practices. Custom
blends can be produced by farm-mixing
where permitted.
Enlarged Internal Organs, Especially of the Spleen and Kidney, seen in Tilapia Infected with F. Asiatica
It is imperative that producers feed the full
regimen of feed medicated with Aquaflor
and that feed medicated with Aquaflor is
fed as the sole ration during the treatment
period. If the farmer is uncertain that the
medication will work, bacterial isolation
and susceptibility testing must be
performed at a diagnostic laboratory.
Skipping treatment days or administering
feed medicated with Aquaflor for fewer days than recommended will lead to
wasted resources. In addition, lower tissue
concentrations of the drug in fish can lead
to the selection of resistant bacteria, and
resistance to one medicated feed can lead
to resistance against other medicated
feeds, eventually leaving the farmer with
fewer options for bacterial treatment.
Alternatively, producers may be tempted
to administer medicated feed excessively
because Aquaflor is highly palatable,
but this will only lead to higher tissue
concentrations than necessary, which
wastes resources. Producers should
adhere to recommended dosages and
required withdrawal times to prevent
prohibitive residues.
Conclusion
Aquaflor is effective against aquatic
warmwater bacterial infections such
as F. columnare, S. iniae, S. agalactiae,
A. hydrophila and E. ictaluri. Fish showing
early signs of disease associated with
these pathogens should be examined
for lesions and cultured at a diagnostic
laboratory to identify the bacteria.
It is imperative that producers use feed
medicated with Aquaflor according to
label directions to ensure maximal
efficacy, preserve bacterial susceptibility
and prevent prohibited tissue residues. When used in conjunction with good
husbandry, vaccines and improved
genetic stock, judicious use of Aquaflor
is a valuable tool for controlling warmwater
fish mortality from bacterial infections.
September 2012
This article contains information on veterinary pharmaceutical and biological animal health products based on international registration dossiers. It may refer to products that are either not available in your country or are marketed under a different trade name. In addition, the safety and efficacy data and the withholding periods for a specific product may be different depending on local regulations. Consult the the regulatory and technical information on available veterinary drugs in your country.



