Identity
Artemia spp Leach, 1819 [Artemiidae]
FAO Names: En - Brine shrimps nei, Fr - Crevettes de salines nca, Es - Artemias nep
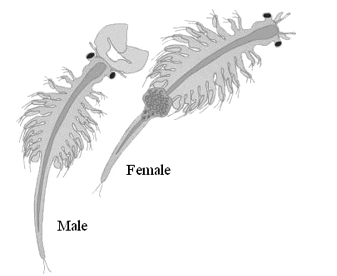
Biological Features
Artemia is a primitive arthropod with a segmented body to which are attached broad leaf-like appendages named thoracopodes, which greatly increase apparent size. Their adult length is ~8-10 mm for males and ~10-12 mm for females but the width of both sexes, including the legs, is ~4 mm. The body is divided into head, thorax, and abdomen. The head consists of one prostomial and five metameric segments which bear in order the median and compound eyes and labrum, first antennae, second antennae, mandibles, first maxillae or maxillulae, and second maxillae or maxillulae. The thorax is constructed of eleven segments, each provided with a pair of thoracopodes, while the abdomen is composed of eight segments. The anterior two abdominal segments are often referred to as the genital segments and of these the first bears the gonopods, either the egg sac of the female or the paired penes of the male. Abdominal segments 2-7 lack appendages. The final abdominal segment possesses the cercopods, also called the furca or telson. The entire body is covered with a thin, flexible exoskeleton of chitin to which muscles are attached internally. The exoskeleton is shed periodically and in females a moult precedes every ovulation, while in the male a correlation between moulting and reproduction has not been observed.
The genus Artemia comprises a number of sexually reproducing species (‘bisexual species’) and a number of parthenogenetically reproducing populations. There are very few macroscopically visible morphological differences between the various species of the genus. The identification of bisexual Artemia species has therefore been established by cross-breeding tests, morphological and morphometrical differentiation, cytogenetics and allozyme studies; presently, increasing importance is being given to nuclear and mitochondrial DNA analysis, including sequencing. With the exception of cross-mating, all these techniques have also contributed to identifying the parthenogenetic types described as A. parthenogenetica Barigozzi (1974). The phylogenetic relationship of populations and/or species within the genus is still a matter of discussion and the need for a multi-trait approach to identify species is generally recognized as essential. The name A. salina has caused considerable confusion worldwide as authors have often named (and continue to name) all brine shrimp A. salina, whereas this species name should be restricted to one of the bisexual species, which is specifically found in the Mediterranean area.
The differentiation of 7 bisexual species, defined primarily by the criterion of reproductive isolation as found in laboratory tests, and of many parthenogenetic populations is currently acknowledged. Endemic to Europe, Africa and Asia (and also found in Australia) are the parthenogenetic populations (with different levels of ploidy). On these continents are also found the bisexuals A. salina, Leach 1819 (Mediterranean area), A. urmiana (Günther, 1890) (Lake Urmia, Islamic Republic of Iran and one Crimean site, Ukraine), A. sinica (Cai, 1989) (inland China and Mongolia), Artemia sp. (Pilla & Beardmore, 1994) (non-defined lake in Kazakhstan), and A. tibetiana (Abatzopoulos et al. 1998) (Tibet). Endemic to the Americas are A. persimilis (Piccinelli & Prosdocini, 1968) (southern South America) and A. franciscana (Kellogg, 1906) (North, Central and South America), with A. franciscana monica being a special case of a population described for an ecologically unique habitat (Mono lake, California, United States of America).
Images Gallery

Profile
Historical Background
The status of Artemia as an economic commodity began in the 1930s when some investigators adopted it as a convenient replacement for the natural plankton diet for fish larvae thus realizing the first break-through in the culture of commercially important fish species. In the 1950s Artemia cysts were still predominantly marketed for the aquarium and pet trade at prices as low as USD 10/kg. There were only two commercial sources: the coastal salt works in the San Francisco Bay (California, United States of America) and the Great Salt Lake (Utah, United States of America). With fish and shrimp operations emerging from the early 1960s onwards, new marketing opportunities were created for Artemia cysts. However, by the mid 1970s increased demand, declining harvests from the Great Salt Lake, high import taxes in certain developing countries and possibly artificial cyst shortages created by certain companies resulted in a severe price rise for Artemia cysts (up to USD 50-100/kg). The dramatic impact of the cyst shortage on the expanding aquaculture industry invigorated research on the rationalization of the use of Artemia and the exploration of new cyst resources. The cyst shortage simultaneously invigorated the search for alternatives for Artemia in an attempt to abandon its use as live food in larval nutrition; a process that continues till today with slow but steady successes.
Harvesting Natural Artemia Resources
During the 1980s improved harvesting techniques and favourable hydrological and climatic conditions enabled a tenfold increase in the yields from the Great Salt Lake source (>200 tonnes of processed product) while the hatching quality was also improved, thanks to an improved understanding of cyst biology. Over the history of its exploitation the Great Salt Lake – however large – remained a natural ecosystem subject to climatic and other influences; this has been illustrated by unpredictable and fluctuating cyst harvests. New insights in hatching characteristics and nutritional essentials gave rise to the segregation of different cyst qualities since the 1980s. Cyst prices thus became quality dependent, ranging from USD 25/kg to USD 80/kg by 1990. At the same time cyst consumption increased exponentially as a consequence of the booming shrimp and marine fish industries. In 1997 some 6 000 hatcheries required over 1 500 tonnes of cysts annually. At that time about 80 to 85 percent of the total sales of Artemia went to shrimp hatcheries, the remainder being used in marine fish larviculture in Europe and East Asia and for the pet fish market; this situation has hardly changed since. While at the end of the previous century, harvests from Great Salt Lake were dramatically low, the situation has returned to normal since then, with annual harvests in the order of 2 000-3 000 tonnes of finished product, making up more than 90 percent of the world’s cyst market. Despite this, the need for alternative resources and the increased demand from aquaculture has resulted in the occasional or regular exploitation of many other small and medium inland salt lakes, especially in southern Siberia, Kazakhstan and China and in coastal areas of the Bohai Bay, China, along with further rationalization in the use of Artemia.
Farming Artemia
Along with the exploitation of natural resources, intensive cyst production in solar saltworks (especially in East Asia and Latin America) comprises an important market share in terms of high product quality and the importance of local cyst production in sustaining aquaculture development in many countries in the South. Often this production is carried out seasonally (e.g. in monsoon Southeast Asia) in areas where there is no natural occurrence of Artemia. This involves the deliberate transplantation of Artemia, not only for the production of Artemia cysts or biomass in itself but also because of the beneficial effect of Artemia presence on the salt production process. High water viscosity in the crystallisers, as created by algal blooms upstream, may completely inhibit salt crystal formation and precipitation. The presence of brine shrimp in sufficient numbers is essential not only for controlling these algal blooms but also for the development of halophilic bacteria in the crystallisation ponds, which proliferate on Artemia decomposition products. High concentrations of these bacteria promote heat absorption, thereby accelerating evaporation, hence crystallisation. Depending on climatological conditions, inoculations can also be considered definitive when one or a few attempts of inoculation will lead to the permanent establishment of an Artemia population, as in Australia and China.
The first attempts in the inoculation and subsequent management of Artemia in solar saltworks was performed in the 1970s in Brazil, soon followed by the Philippines, China and Thailand. However, it is mainly in Viet Nam that this activity has proven particularly successful. Since the first initiatives of the 1980s interest in the seasonal culture of Artemia in the Mekong Delta aimed at cyst production has expanded and the know-how has gradually been transferred to artisanal salt farmers via local cooperatives. This alternative farming system has been increasingly successful and has resulted in higher profits for salt farmers compared to their traditional low income from salt production alone. In 1990, about 1.4 tonnes of raw cysts were collected from a culture area of 16 ha, which made the product available for commercialization. By 2001, the production area had increased to >1 000 ha of salt-fields in the Vinh Chau and Bac Lieu coastlines, yielding almost 50 tonnes of raw cysts. This region is currently an important supplier of high quality cysts for domestic use and for the international market.
The nutritional value of Artemia cysts varies highly between geographical sources, especially in the level of essential highly unsaturated fatty acids, and also from batch to batch. Hence appropriate techniques (‘enrichment’ or ‘bioencapsulation’) have been developed to improve the hatchery use and maximize the nutritional value of Artemia nauplii. These techniques take advantage of the indiscriminate filter-feeding behaviour of brine shrimp and use them as a vehicle for administration of selected fatty acids, vitamins, essential nutrients and therapeutics to fish and shrimp larvae. This and other developments, such as cyst decapsulation and nauplius cold storage techniques, have contributed to the fast expansion of the industrial farming of an increasing number of aquaculture species globally. Finally, although Artemia is mostly used in the form of freshly hatched nauplii, more and more use is made of juvenile and adult Artemia (known as biomass) in shrimp nursery and maturation facilities.
Main Producer Countries
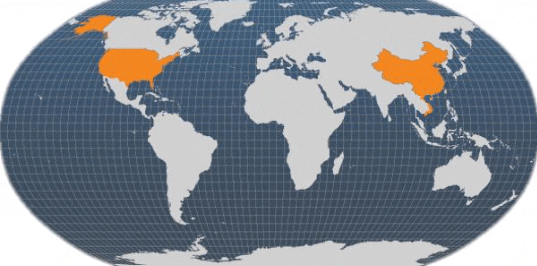
Main producer countries of Artemia cyst (Van Stappen, 2012)
Habitat and Biology
The brine shrimp Artemia (Crustacea, Anostraca) is a zooplanktonic organism found globally in hypersaline habitats such as inland salt lakes, coastal salt pans and man-managed saltworks. Presently more than 600 sites have been recorded, although such lists reflect systematic inventory work for specific areas, rather than an accurate reflection of true zoogeographical distribution, since many areas (e.g. sub-Saharan Africa) remain under-explored. No Artemia is found in areas where year-round low temperatures exclude its development, but a lot of strains are found in the continental areas of North and South America and Asia with extremely cold winter temperatures, as long as sufficiently high summer temperatures allow cyst hatching and subsequent colonization of the environment.
Being extremely osmotolerant, brine shrimp survive in environments with salinities ranging between approximately 10 and 340 per thousand with diverse ionic composition and temperature regimes; in general the lower salinity threshold of its occurrence is determined by the salinity tolerance of its predators in the area, and abundant Artemia populations are consequentially only found at salinities elevated enough to eliminate (nearly) all predators or food competitors. Artemia is exceptionally adapted to such extreme environments, due to its unique osmoregulatory capacity and its capacity to synthesize highly efficient haemoglobins. Artemia reproduces by two modes, involving either nauplius (ovoviviparous) or cyst (oviparous) production, depending on the prevailing ecological conditions. Ovoviviparity occurs under favourable ambient conditions: eggs (fertilized following mating in the case of bisexual species or non-fertilized in the case of parthenogenetic females) produce free-swimming larvae (‘nauplii’) released by the mother. On the other hand, oviparous reproduction occurs under unfavourable conditions usually characterised by factors such as high salinity, low oxygen levels, temperature stress, food depletion, etc. In this mode, the embryos only develop up to the gastrula stage and become surrounded by a thick shell (chorion) induced by hormonal secretions of the brown shell glands located in the uterus, thus forming what is referred to as a cyst. The embryo enters a state of metabolic arrest described as diapause and is spawned by the female. Both oviparity and ovoviviparity are found in all Artemia strains, and female individuals can switch from one mode to the other between two reproduction cycles.
In nature, cysts may be produced in massive numbers, and the alveolar structure of the chorion ensures that large quantities float on the water surface, or may eventually be blown ashore by wind and waves. Upon dehydration, often in combination with other environmental cues, cyst diapause is deactivated, giving quiescent embryos with the ability to resume further embryonic development when hydrated in optimal hatching conditions. Once harvested and properly processed, the cysts can be stored for several years while the dried embryos stay in a state of arrested metabolism. When quiescent cysts are immersed in lower salinity water, the biconcave cysts hydrate, becoming spherical and the shelled embryo resumes its interrupted metabolism. After a few more hours (depending on ambient conditions and strain) the cyst outer membrane breaks and the embryo appears, surrounded by a hatching membrane. At this point (umbrella stage) the embryo hangs underneath the empty shell, the development of the nauplius is completed and, within a short period of time, the hatching membrane ruptures (hatching) and the free-swimming instar I nauplius is born. This larva can be used as it is or, following a specific enrichment procedure to enhance its nutritional properties, as a convenient substitute for the natural plankton diet of fish and shrimp larvae. Under favourable ecological conditions, Artemia can live for several months, growing from nauplius to adult in only eight days and reproducing at up to 300 nauplii or cysts every four days.
The bulk of the Artemia product reaching the world market is A. franciscana from the Great Salt Lake; product from continental Asia consists of a variety of parthenogenetic strains and A. sinica. Harvests resulting from seasonal production in solar saltworks (such as in Viet Nam) generally belong to the San Francisco Bay-type A. franciscana, as this strain has been used for the original inoculation material. Depending on climatological conditions, an allochthonous strain may establish itself following deliberate or non-deliberate introduction by man. Recently the gradual dispersion of A. franciscana into new environments competing with and eventually out-competing local populations is becoming an increasingly common pattern in various parts of the world. Its fast growth and reproduction and its high temperature and salinity resistance, combined with its attractiveness for aquaculture applications (e.g. small cyst size and simple diapause termination procedures in the case of San Francisco Bay A. franciscana) explain its popularity as an inoculation strain in saltworks. Moreover, A. franciscana from the Great Salt Lake is the dominant strain used in hatcheries worldwide. Unhatched cysts and un-consumed nauplii may be drained into the wider environment together with hatchery effluents. A growing number of field observations worldwide (Mediterranean area, India, East Africa, Australia, coastal China, etc.) hint at the emergence of A. franciscana in new coastal environments, thus contributing to the complexity of the species status of cyst product originating from these areas. Product harvested from the Bohai Bay area, China, for example, may thus consist of variable mixtures of parthenogenetic strains (the autochthonous coastal populations), originally inland Chinese A. sinica (dispersed coastally following its use in local aquaculture farms) and allochthonous A. franciscana (deliberately introduced; non-deliberately dispersed following its use in local aquaculture farms).
All Artemia product reaching the market is produced from feral strains (such as Great Salt Lake) or from feral populations that have adapted following their introduction by man in a new environment (such as the Vietnamese saltworks). So far no fully-fledged Artemia breeding or selection programmes have been launched, though research work is being done on the heritability of commercially interesting characteristics (e.g. synchronous hatching rate, small cyst size). Moreover, knowledge on genotype-phenotype relationships in Artemia is steadily growing as it is increasingly and intensively being used as a model organism in aquaculture breeding programmes, focusing e.g. on disease resistance and stress tolerance.
Production
Production Cycle
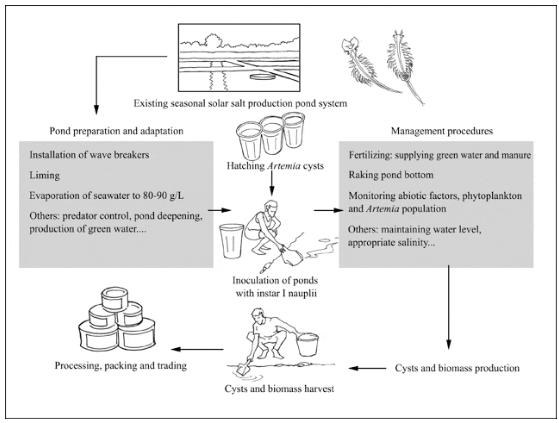
Production cycle of Artemia
Production Systems
As the bulk of the Artemia cysts entering the world market originates from Great Salt Lake, Utah, United States of America, and as local harvesting procedures and regulations are strictly defined and publically known, information on the harvesting procedures at this site are described in detail below.
Compared to the Great Salt Lake, harvesting regulations in other salt lakes that are inland (e.g. Russian Federation, Kazakhstan, China) are generally much less well defined. Although there may be a governmentally imposed quota system, the vastness and remoteness of the territory and the variety of lakes harvested make it difficult to eliminate all illegal harvesting. Generally the harvesting technology in these lakes is determined by:
- Accessibility and topography of the site (cysts may be partially or primarily collected from the shores if there are no means to access open water).
- Harvestable quantities.
- Duration of the harvesting season.
- Characteristics of the local brine shrimp population.
- Financial means for investment in harvesting logistics and infrastructure.
Cysts and biomass are also harvested in coastal saltworks; this may be the result of natural productivity (i.e. no or minimal human intervention to enhance productivity) or of intensive management procedures, as determined in detail for seasonal solar saltworks in the Mekong Delta, Viet Nam. The latter system is also described below.
On the other hand the production of Artemia biomass in indoor recirculation systems, which used to be practiced in limited amounts for the benefit of niche markets (e.g. the pet market), is of virtually no economic importance anymore and is therefore not described below.
Cyst Harvesting at Great Salt Lake
The brine shrimp harvest on the Great Salt Lake is an annual event which is regulated and monitored by the Utah State Division of Wildlife Resources. The State currently issues 79 permits or Certificates of Registrations (COR) to brine shrimp companies. Due to the State only issuing a limited number of COR, the barriers to entering brine shrimp harvesting are very severe. Brine shrimp companies pay around USD 12 000/year/COR. On top of these fees, the harvesting companies must also pay State royalties on the total ‘raw harvest’. The ‘raw harvest’ includes brine shrimp, brine shrimp eggs, empty shells, brine flies, brine fly casings, algae and other biomass material. In 2006, most of the members of the brine shrimp industry grouped together to form the Great Salt Lake Brine Shrimp Cooperative. The Cooperative harvests, processes, packages, markets and sells brine shrimp globally.
The harvest begins on 1 October each year and continues until no later than 31 January. The harvest is monitored by the State and the harvestable quantities are calculated based on the cyst density in the lake, which are followed through a continuous population monitoring programme, and on the minimal inoculum of cysts estimated for colonization of the lake (and thus sustainability of the population) for the following year. If the cyst count falls below a certain level the harvest may be suspended for a week or even closed for the year. The harvesting season is also closed as soon as the total harvestable quantities have been collected. Several conditions affect the availability of cysts from year to year. Natural conditions (temperature, salinity) either indirectly (through their effect on primary production) or directly determine the size and reproductivity of the Artemia population. Salinity is crucial for the harvest: at lower salinities (e.g.
Competition during the harvesting period is fierce. Spotter planes, GPS, and night-vision technology are used to locate large accumulations of cysts. The procedures to claim a cyst accumulation spot as company property are strictly defined. The harvesting fleet comprises fast chase boats (used to claim cyst spots), the haul boats that the raw cyst material is loaded on, and boom boats carrying an oil containment boom to corral the cyst mass. Once corralled the floating cyst mass is pumped into porous super sacks from which excess water is drained, and is brought ashore.
Cyst and Biomass Production in Solar Saltworks
Controlled Artemia production is carried out in coastal salt works where seawater is concentrated by evaporation until crystallization. Artemia can be cultured in permanent solar salt operations and seasonal artisanal units. In both systems Artemia are mainly found in ponds at intermediate salinity levels since Artemia has no defence mechanisms against predators (generally above 80-100 per thousand; at salinities exceeding 200-250 per thousand brine shrimp productivity tends to decrease).
Seasonal Units
Seasonal units are small artisanal salt works in the tropical-subtropical belt that are only operational during the dry season. Typically, ponds are only a few 100 m2 in size with depths of 0.1 to 0.6 m and generally are only operated for a few months, when the balance between evaporation/precipitation is positive. Usually, salt production is abandoned during the rainy season, when the evaporation ponds are often turned into fish or shrimp ponds. In seasonal Artemia culture systems production takes place mostly in static systems where ponds are independently managed. These systems consist of a reservoir, a fertilization pond and Artemia ponds in respective proportions of 20 percent, 25 percent and 50-60 percent; the exact ratios vary according to the region, tidal regime and the water level of reservoir and fertilisation ponds.
Permanent Units
Permanent solar salt operations typically consist of several interconnected evaporation ponds and crystallizers, where ponds may each be a few to several hundred hectares with depths of 0.5 to 1.5 m. Seawater is pumped into the first pond and flows through the successive evaporation ponds; meanwhile salinity levels gradually build up as a result of evaporation. Due to their size and their quasi-permanent operation, this type of saltworks often involves a higher degree of mechanisation compared to the small seasonal units.
Site Selection
Site selection and pond design should meet a number of criteria. Ideal pond soils should be clayish and limit seepage. As in fish ponds, Artemia production ponds are designed with water inlet and outlet canals that facilitate filling and draining with high salinity water or freshwater. Salt ponds are modified for Artemia production by deepening the ponds to 40-50 cm in regions with high temperatures, which may become stressful for the Artemia. Deepening involves digging a peripheral ditch and using the excavated earth to heighten the dikes. Screens should be installed to prevent predators from entering the culture ponds, while wave breakers prevent foaming and also act as cyst barriers to facilitate cyst harvesting. Suitable Artemia production ponds range in size from 0.05 to 0.5 ha.
Pond Preparation and Management
Pond preparation begins after the end of the rainy season when all ponds are drained and pond and canal bottoms are scraped, followed by sun drying for about one week. Liming is applied for acidic soils. For optimal productivity, proper pond management must be ensured by maintaining optimal salinity (80-100 per thousand) and temperature (
Harvesting in Solar Saltworks
Cysts released by females float on the water surface and are normally prevented from washing onto the dykes by wave breakers made from plastic sheets or bamboo. These wave breakers are placed at corners at the leeward side of the pond and aid in cyst collection. Cysts floating in the corner are collected by dip nets of appropriate mesh size (e.g. 150 µm), normally every morning depending on production level. For collecting adult Artemia a 1 mm mesh net is used after three weeks of culture, especially in the morning or afternoon when most individuals are found near the water surface. Harvesting frequency may depend on need, as it can be done daily or twice a week with biomass harvests of 25 to 50 kg ww/ha/day, with production levels as high as 0.7 to 1 tonnes/month over a culture period of four to five months. Guidelines for proper biomass harvesting periodicity and quantities have been developed, ensuring optimal biomass productivity over the entire production season and compatibility with cyst production requirements.
Handling and Processing
Depending on local conditions, the quality/quantity characteristics of harvests and the business structure of harvesting companies, processing may be carried out partially on-site, and/or the product may be sent for domestic or overseas transport for final or complete processing elsewhere. A number of consecutive processing steps are carried out in order to obtain a clean, marketable product featuring acceptable hatching parameters and shelf life. The processing steps aim firstly at purification of the raw material (i.e. eliminating as much as possible all fractions which are not full cysts), and secondly dehydration in order to reduce water content to the range 5-10 percent that is necessary for long-term storage. Processing generally implies sequential combinations of mechanical sieving, brine washing/dehydration and drying, combined with one or more storage periods in specific conditions of temperature and water content, in order to break diapause. Organization, logistics and scale of the processing line may vary and are determined by the trade-off between required final quality, processing quantities, specific strain/batch characteristics, local conditions (i.e. site location, storage facilities, local equipment available) and financial imperatives. Throughout processing, rigorous quality control is implemented in order to adjust or correct the techniques and to obtain a final product of good and constant marketable quality.
Production Costs
Because of the highly proprietary nature of Artemia production, no cost data is publicly available.
Diseases and Control Measures
In laboratory cultures of Artemia infestation with Leucothrix bacteria and the so-called black spot disease have been observed. The latter disease has also occasionally been observed in Great Salt Lake adult Artemia, and is generally linked with dietary deficiency caused by unusual phytoplankton abundances and species composition, being the consequence of highly aberrant abiotic conditions. The pathogenicity of several Vibrio species for A. franciscana has been demonstrated in laboratory cultures. Cestode parasitosis, with Artemia being an intermediate host for water birds predating on them, has been studied in natural Mediterranean brine shrimp populations. However, as Artemia production is essentially the harvest of a natural resource (albeit sometimes in man-managed conditions such as in solar saltworks), information on overall incidence of infestations is fragmentary at best, and issues such as the effect on population productivity and possible control measures are highly speculative.
Statistics
Production Statistics
Although the existence of aquaculture (farmed) production of Artemia in Australia, Bahamas, Madagascar and Peru is recorded in FAO aquaculture production statistics, no quantitative data is currently available (up to 2009).
The following information refers to available statistics on certain specific capture fisheries for Artemia from non-FAO sources. Reliable capture fisheries production statistics are only available for Great Salt Lake and for production in the Mekong Delta, Viet Nam, where production is systematically monitored by local authorities or scientific organizations. In the absence of such public monitoring bodies in other areas, data on production lack completeness and/or reliability or are not openly accessible.
Production Statistics from the Great Salt Lake
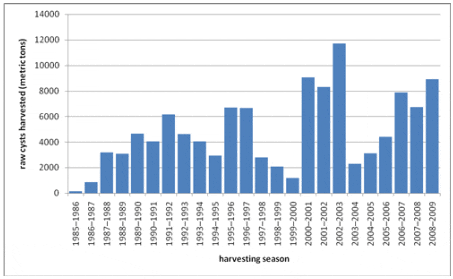
Source: State of Utah Division of Wildlife Resources, Great Salt Lake Ecosystem Program
The graph reproduced above shows total weight (tonnes) of raw biomass harvested as reported by the harvesting companies. Raw biomass includes cysts, empty shells, Artemia biomass, algae and other material. The yield of dry processed cysts from the raw biomass varies annually, but is typically 30-35 percent.
Culture Area, Total Production and Yield of Artemia Cysts (raw product) in the Vinh Chau and Bac Lieu Districts of the Mekong Delta, Viet Nam
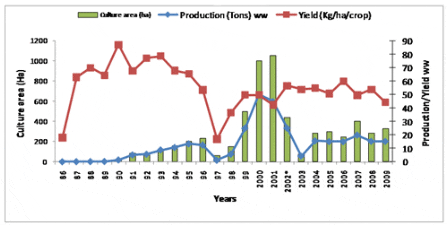
Source: College of Aquaculture & Fisheries, Can Tho University, Viet Nam
Market and Trade
Artemia biomass
Artemia biomass is rich in protein, lipid, attractants, pigments and other active substances making it an attractive direct feed for ornamental fish or an excellent ingredient for aquafeeds, e.g. as a maturation trigger for shrimp broodstock and for young juveniles of marine fish, shrimp, crabs and new aquaculture species. Ensilage or deep-freezing has been used, but for incorporation in aquafeeds, Artemia biomass is generally simply dried. Fresh Artemia biomass contains a very high level of moisture (approximately 90 percent) and is rich in proteolytic enzymes; consequently it decomposes quickly and is difficult to store and transport, and is subject to rapid quality deterioration. In the Mekong Delta, large amounts of Artemia biomass, a by-product from commercial cyst-oriented Artemia production, can be collected at average rates of 0.2-0.3 tonnes/ha when the culture season ends. It is expected that interest in this product will continue to increase (e.g. Artemia biomass production as a form of extractive aquaculture to reduce nutrient loads in effluents) and culture procedures are being developed that focus on maximal biomass production as an alternative to cysts.
Artemia cysts
Artemia cysts are generally marketed vacuum packed and dehydrated to 5-10 percent; these storage conditions guarantee maximal viability. Cysts are offered on the market in a number of brands, corresponding with a variety of quality criteria, amongst which hatching quality, cyst size (and hence naupliar biomass) and nutritional composition (HUFAs, vitamins) are among the most important. These criteria, as well as practical aspects related to their daily manipulation in the hatchery (decapsulation behaviour, ease of nauplii harvesting, nauplius colour, enrichment kinetics, etc.) and microbiological aspects all contribute to the sale prices of each brand. Most countries require cysts to be imported with a veterinary certificate, declaring absence of pathogens such as Salmonella and Enterobacteriae.
Whereas there have been large fluctuations on the offer side over recent decades, there has been a steady increase on the demand side due to the global increase of aquaculture activities. Cyst prices have fluctuated considerably over the last few decades as a result of fluctuating Great Salt Lake harvests, and of the variable market availability of cysts from alternative geographical locations. On the demand side, prognoses about the future demand for Artemia cysts are speculative, because this is determined by divergent parameters such as:
- The overall expansion and intensification of production of current aquaculture species.
- The emergence of new aquaculture species.
- Dietary and zootechnical improvements resulting in a more efficient use of Artemia nauplii.
- The trend towards co-feeding, early weaning diets and Artemia substitutes.
Since 1990 cyst consumption has increased exponentially as a consequence of the booming shrimp and marine fish farming industries. In 1997, some 6 000 hatcheries required over 1 500 tonnes of cysts annually. Some 80 to 85 percent of the total sales of Artemia went to shrimp hatcheries, mainly in China and South East Asia as well as Ecuador and a few other Latin American countries. The remainder went to marine fish larviculture in Europe, China and Japan as well as to aquarium fish producers.
On the other hand, rationalization of the use of Artemia in hatcheries has enabled a dramatic reduction in the amount of cysts needed to produce each unit of fish or shrimp, and Artemia costs presently comprise ~15 percent of hatchery costs; this compares to 25-50 percent (depending on the species being fed) before rationalization. For instance, in 1990 a typical Mediterranean seabass and seabream hatchery would have been using some 600-700 kg of cysts to produce 1 million fry while in 2011, the required amount has been reduced to
Reliable estimates of future supplies remain difficult to obtain due to the lack of information on the ecology of new sites, the productivity of the local Artemia population, and on technical and economical studies related to accessibility, sustainable quantities, characteristics of the resource relevant to larviculture, etc. Therefore, no stable cyst provision can be guaranteed; diversification of resources remains a most important issue, along with the further rationalization of the use of Artemia. As a net result of all the factors that impact the aquaculture market, the global demand for cysts, 2 500-3 000 tonnes/year, is expected to increase further. China is the main consumer (and will continue to be so) with an annual consumption of 1 500 tonnes, of which about one half is imported from Russian Federation and Kazakhstan and the other half produced domestically (e.g. from the Bohai Bay area, which has a relatively stable annual production of about 400 tonnes of raw product). Only a small quantity (
Status and Trends
As harvests at Great Salt Lake essentially depend on fluctuating natural productivity and are subject to quota, further efforts will be made to explore alternative resources and to further expand and consolidate knowledge on alternative biotopes, the productivity of local Artemia populations and how these strains can be applied in aquaculture with maximal efficiency (e.g. optimal diapause deactivation strategy, enrichment kinetics, decapsulation behaviour, etc.). At the same time, though important technological advances have been realized in Great Salt Lake harvesting procedures, it is expected that further rationalization of harvesting and processing procedures will take place. Linked with this further diversification of resources, it is expected that the present trend of diversification of cyst products on the market, targeting specific application aspects (different naupliar characteristics such as size and nutritional profile, microbiological aspects, hatching behaviour, separation of nauplii from empty cyst shells, decapsulation behaviour, etc.), will continue. Improvements related to general organizational issues such as reduction in manpower, space requirements for live food production, the smoothing out of occasional variations in live food quality, etc., are also constantly a focus of cyst producers.
In Viet Nam, the initial expansion in the 1990s, thanks to the empirical development of the technique, and the dissemination of knowhow to a wider group of salt farmers, encountered some limitations shortly after the turn of the 21st century; this was reflected in lower yields, total production and culture area. This is the result of a combination of factors, such as:
- A significant increase in production area without appropriate planning.
- The superficial knowledge of the farmers newly joining the activity.
- Large variations in the socio-economic background of the farmers and hence the economical effect within the region.
- The instability of the output market.
- The effect of occasional unusual weather conditions.
Further consolidation of pond production technology, which is currently highly empirical, to a fully scientifically sustained activity, combined with permanent and thorough dissemination of knowhow to the artisanal salt farmers, is a prerequisite for the really sustainable development of Artemia production in this region. Apart from cyst production, more emphasis will be given to Artemia biomass production (as a replacement for more traditional aquafeeds) thus providing more flexibility in integrated systems, and culture systems are being developed to make production of both products compatible. This evolution will be in tune with concurrent local developments in aquaculture - increasing diversity of highly economic cultured species requiring excellent quality foods. In parallel, it is expected that Artemia production will provide an additional benefit as a means of extractive aquaculture (the removal of excess nutrients through the Artemia grazing on phytoplankton, detritus and bacteria from effluents from - but not exclusively - aquaculture activities.
Consolidation of the technology would also benefit from a governmental policy in Viet Nam favourable for Artemia production (such as exists, for example, for aquaculture development); this is presently still lacking, probably because the gross output of the industry is limited compared to the total national or regional GNP. As a consequence, the industry has remained beyond the scope of economic analysis and modelling; it is only recently that the first econometric analysis of integrated Artemia production in the Mekong Delta has been performed. This has resulted in the formulation of recommendations to increase the production efficiency from an economic point of view, and hence to improve livelihoods.
Globally, it is expected that the model developed and the experience gained in Viet Nam will be used for similar initiatives in other areas, albeit with the necessary adaptations, either as an entirely new activity (e.g. in sub-Saharan Africa) or by enhancing the economic value of Artemia production through improved scientific management in areas where it has already been introduced (e.g. Bohai Bay area, China).
Main Issues
The ubiquitous application of Artemia as a live food in hatcheries has raised a number of issues related to its future use:
- Replacement of Artemia by artificial diets. Almost as long as Artemia has been used as a primary live food source in hatcheries, its full replacement by artificial diets has been predicted. However, this has remained wishful thinking until now. Progressive rationalization of its use is continuously taking place and replacement is sometimes possible to a large extent thanks to early weaning and co-feeding rearing procedures, for example. However, it is generally expected that Artemia will remain an essential economic commodity due to the continuous trend for species diversification in aquaculture (attempts to culture larvae of ‘new species’ are generally made with live food) and to the biological limitations inherent in the reduction of live food during early marine fish and shrimp rearing.
- User-friendliness of commercial Artemia cyst products. As the use of Artemia cysts in hatcheries requires a lot of labour, the manipulations needed to produce the live food from the cysts are a focal R&D topic for the cyst producing companies. The fact that a product or method allows rationalization of procedures such as hatching, nauplius harvesting, and nauplius storage, is generally used as an important sales argument.
- Microbiological aspects of the use of Artemia as live food in hatcheries. The increasing awareness of the importance of the microbiological aspects of larviculture has resulted in a higher alertness to the role of microbiological communities associated with the use of live food, such as algae, rotifers and Artemia. This includes the enhancement of communities that have a positive impact on culture performance and the reduction or elimination of communities that have a detrimental effect. Already in the 1980s the technique of decapsulating cysts before hatching had been developed with the aim of reducing the bacterial load in the hatching medium, and it has been widely adopted since. The research and development of cyst products claimed to have a favourable effect on the microbiological live food environment are highly proprietary. The role of Artemia as an external or internal carrier of pathogens into larviculture tanks (and the procedures to minimize or neutralize this risk) is currently a focus of intensive research.
Responsible Aquaculture Practices
Public environmental debate about Artemia production issues is limited to the Great Salt Lake operations, where a balance is sought between the needs of the Artemia cyst production industry and the importance of the Artemia resource for birds. Great Salt Lake is a key foraging site for migrating avifauna, and A. franciscana is a key species upon which much of the rest of its ecosystem relies. Proper management and maintenance of the brine shrimp resource is carried out through continuous monitoring of the population under the supervision of the Utah Division of Wildlife Resources and the quota system imposed by the authorities ensures the sustainability of the resource, in line with article 7 ‘Fisheries Management’ of the FAO Code of Conduct for Responsible Fisheries.
With the exception of Urmia Lake, Islamic Republic of Iran, where harvest control was exercised by environmental authorities but where the Artemia population has dwindled over the last few years due to extremely high salinity (hence cyst harvesting has stopped), there is little information about the ecological importance of other lakes (Russian Federation, Kazakhstan, China) where Artemia is commercially harvested. Because governmental control on harvests at these sites may be insufficient or absent, it is unlikely that wildlife conservation is considered an issue in those locations.
Environmental concern may also be raised by the reduced Artemia biodiversity due to the introduction of allochthonous strains in coastal saltworks. Artemia pond production is generally practiced in existing man-operated hypersaline systems (coastal saltworks) so its production does not require extra space in an environment that is already often subjected to the pressure of multiple uses (see article 10 ‘Integration of fisheries into coastal area management’ of the FAO Code of Conduct for Responsible Fisheries). The fertilization scheme in intensive Artemia pond production should be designed such that a phytoplankton boom can be induced that can be grazed away by the brine shrimp population, thus limiting the risk of additional eutrophication in line with article 9 ‘Aquaculture development’ of the FAO Code of Conduct. However, Artemia pond production often implies the inoculation of superiorly productive allochthonous strains (generally A. franciscana) in environments where local strain(s) is/are occurring. If a permanent A. franciscana population can establish itself (which depends on climatological conditions), it tends to outcompete the local population(s), as has been demonstrated, for example, in the Mediterranean and in the Bohai Bay area of China. Preservation of the gene pool of the local population in cyst banks is therefore highly recommended.
September 2012
In some cases antibiotics and other pharmaceuticals have been used in treatment but their inclusion in this table does not imply an FAO recommendation.




