Identity
Sander lucioperca Linnaeus, 1758 [Percidae]
FAO Names: En - Pike-perch, Fr - Sandre, Es - Lucioperca

Biological Features
Body elongate, the snout pointed, head length greater than depth of body or equal to it. Upper jaw extends past eye level, small teeth in jaws and several large fangs in front also (never more of 18 branched rays). Two dorsal fins, the first spiny and separated by a narrow interspace from the second. Anal fin with 2-3 spines and 11-13 soft rays. Pelvic fins widely spaced, the distance between them almost as great as the base of one fin. Lateral line with 84-95 scales. Colour greenish-grey or brown on the back and sides becoming lighter on the lower sides and white on the belly. Young fish have 8-10 indistinct dusky bars on the sides; these are faint in the adult. Dorsal and tail fins dark spotted.
View SIDP Species fact sheet
Images Gallery
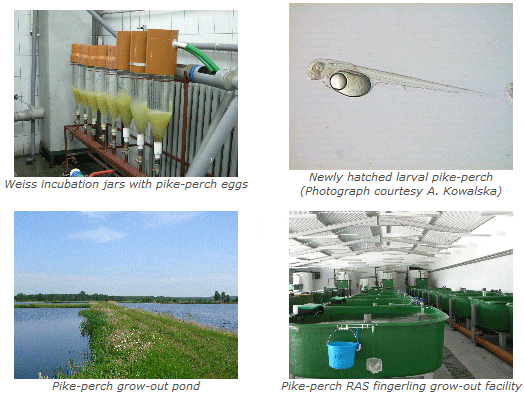

Profile
Historical Background
The beginnings of pike-perch culture date to the nineteenth century and are linked to carp (Cyprinus carpio) culture in earthen ponds in Central and Eastern Europe. Pike-perch was produced in insignificant quantities as a so-called additional fish. In the early twentieth century, production began of pike-perch stocking material (summer and fall fry) in earthen ponds (natural spawning) for stocking open waters. It was produced in monoculture (summer fry) or in polyculture with carp (fall fry). Pond pike-perch culture also began to develop in Western Europe (e.g. France) in the second half of the twentieth century. This type of pike-perch production has been and remains extensive in character, and this species has been and currently is viewed only as a supplementary fish.
At the beginning of the twenty-first century, the first aquaculture facilities producing pike-perch in recirculation aquaculture systems (RAS) were established in Western Europe and, by the close of the first decade, there were less than ten of these facilities. Methods for intensive pike-perch culture are in the initial stages of development but this species is considered to offer good prospects for European aquaculture.
Main Producer Countries
Currently, the main producing countries are the Czech Republic, Denmark, Hungary, Romania, Tunisia and Ukraine. In addition to the other countries shown on the FAO map, pike-perch are also grown in the Netherlands and Poland.
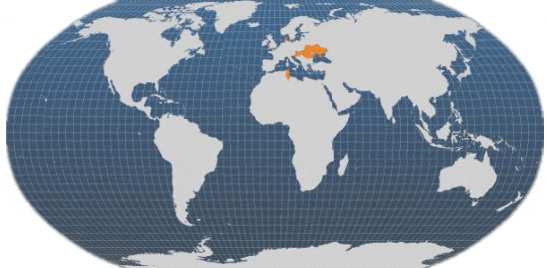
Main producer countries of Pike-perch (FAO Fishery Statistics, 2009)
Habitat and Biology
Pike-perch inhabits lakes, rivers, reservoirs and the coastal marine waters (in the catchment areas of the Caspian, Aral, Baltic, Black, and North seas. It is now widespread in France and western Europe, is rapidly extending its range in eastern and central England, and is acclimated to the waters of northern Africa (Algeria, Morocco, Tunisia), North America, and Asia (e.g. China, Kyrgyzstan).
This species generally attains lengths of 50-70 cm and body weights (BW) of 2-5 kg but a maximum length of 130 cm and weights of 12-18 kg have been reported. Males reach sexual maturity at 2-3 years, females at 3-4 years. Depending on geographical zone, spawning is from April to mid June. Water temperature at spawning initiation ranges from 8.0 to 15.0 °C. Generally, water depth at natural spawning grounds ranges from 0.5 to 3.0 m. Pike-perch deposit eggs into nests that they have built on sand, gravel (preferred substrate), or aquatic vegetation. Males actively guard nests with eggs for 5-8 days until the larvae hatch. Relative fecundity is 170-230 eggs/g BW. Eggs are small; the diameter of unhardened and hardened eggs range from 0.6-1.0 mm and 0.9-1.6 mm respectively. One kg comprises 1.5-2.2 million (unhardened) or 1.0-1.5 million (hardened) eggs. Incubation time is from 3 days (20 °C) to 11 days (10 °C) (80-120 °D). Incubation time for pike-perch eggs (from fertilization to larval hatching) can be calculated with the formula: I = 30 124 × T-2,07, where: I – incubation time (h), T – water temperature (°C).
Larvae are small and devoid of pigment: BW – 0.4-0.5 mg and body length TL – 4.0-5.5 mm. Resorption of yolk sac reserves and lipid droplet is completed at TL 5.8-6.5 mm. Scales begin being laid down at TL 23-28 mm (initially on the caudal peduncle). Pike-perch are regarded as having three trophic phases: plankton phase until fish are TL 13-30 mm; mixed feed stage (invertebrate fauna + fish) until fish are TL 24-70 mm; and predatory phase (feed comprised exclusively of fish) from TL 34-80 mm.
Production
Production Cycle
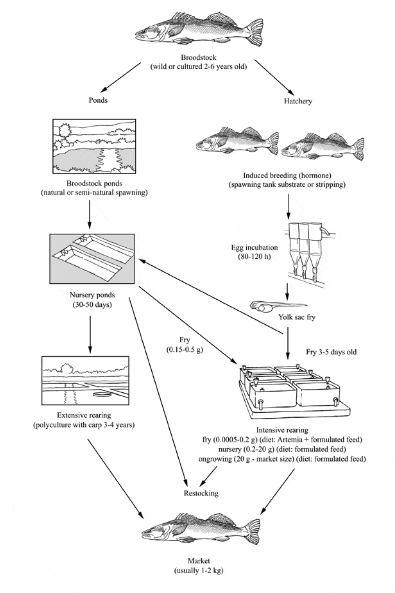
Production cycle of Pike-perch
Production Systems
Seed Supply
Spawners are obtained mostly from natural waters. Catches are made in fall (October-November; summer seine) or spring (March-April; trap gear, e.g. fyke-nets). Fish caught in the fall are held in earthen ponds. For each 1 kg of spawners there should be 10 m² of pond bottom and 1.5-3.5 kg of fodder fish. Spawners should be removed from the ponds when the mean daily water temperature is 8.0-9.0 °C and transported to spawning ponds or hatcheries. A few farms keep broodstocks, usually numbering 50-80 individuals/ha, which are kept in earthen ponds. A few farms produce pike-perch in RAS, and keep spawners in these systems (initial stages of domestication).
Pike-perch do not exhibit distinct sexual dimorphism. Males usually exhibit breeding coloration in the pre-spawning period when they are darker than females. Females have distinctly more distended abdomens. Females with body weights of 1.5-4.0 kg and males of 0.8-2.0 kg are recommended for artificial spawning. Pike-perch spawners should be transported in tanks with aeration. If the transport time is les than 2 hours and the water temperature is 8.0-15.0 °C in 1 m³ tanks, a maximum of 60 kg of fish can be moved. During transport it is recommended that anti-stress agents such as table salt (5 g NaCl/litre) are applied.
Several methods of pike-perch reproduction are used:
- Uncontrolled natural reproduction. One set of spawners (spawner set – 1 ? + 2 ?) per 1-4 ha is released into earthen carp ponds. After spawning, the fish are left in the pond for 6-8 weeks until stocking material - summer fry - has been obtained.
- Controlled natural reproduction. Smaller earthen ponds are used (storage or wintering ponds) with surface areas of approximately 500 to 1 500 m² and depths of 1.5-2.0 m. Spawning nests (60 × 60 cm) are placed on pond bottoms 3-5 m apart. The substrate can be turf, sea grass, rice straw, or alder or willow roots. There should be 10 percent more nests than males. There should also be 10 percent more males than females. Nests with eggs are transported to a hatchery or another pond.
- Reproduction in lake cages. In Finland, cylindrical floating cages with a (diameter 2.0 m; depth 2.0 m) are used. In Poland pens are cubic with sides measuring 2.0 m. Spawning substrate is placed in the cages (as above) along with spawning sets. The females are injected hormonally, usually with human chorionic gonadotropin (hCG) or carp pituitary extract (CPE). Males usually do not require hormonal stimulation. Nests with eggs are moved to ponds or hatcheries.
- Artificial reproduction. After being transported to the hatchery, the fish are sorted by sex, and females by their degree of maturity. A catheter is used to sample eggs, which are then fixed in Serra's fixative to assess the position of the nucleus (on a 4-degree scale). Females are stimulated with hormone injections (hCG – 200-600 IU/kg BW or CPE – 2-5 mg/kg BW). Males receive half of the dose applied to females. After the sex products are obtained, the eggs are dry fertilized (1-2 ml semen/100 g eggs). Adhesiveness is removed from the eggs by rinsing them in an aqueous solution of tannin at a concentration of 0.5-1.0 g/litre water, bath time 2-5 min. Enzymatic preparations can also be applied – 0.5 percent aqueous protease solution (2 min for adhesive removal). Eggs are incubated in Weiss jars. Prophylactic baths are used to prevent the development of mould (e.g. 100 ppm formalin for 5 min).
- Out-of-season reproduction. This is the newest method, and it is used at hatcheries equipped with RAS and cooling systems for reducing water temperatures. Fish are stimulated environmentally (temperature and photoperiod). Thermal stimulation lasts for 18 weeks – 8 weeks cooling phase (20-8 °C), 6 weeks chilling phase (8-4-8 °C), 4 weeks warming phase (8-12 °C). Photoperiod stimulation is used exclusively during the warming phase when it is changed from 8L:16D to 14L:10D. After this period, hormonal stimulation is used (see above). This permits obtaining sex products several months before the natural spawning period.
Hatchery Production
Two types of hatchery production are employed, an extensive/intensive method and a solely intensive method. Hatchery-reared seed is not normally used for producing juveniles to be reared in ponds.
Extensive/intensive method (ponds ? RAS)
Larvae are reared in earthen ponds in which natural or semi-natural pike-perch reproduction has previously been conducted. The optimal surface area of rearing ponds for larvae is 0.5-2.0 ha, with a mean depth of 1.2-1.5 m. Organic fertilization is applied - manure (5-8 tonnes/ha). Depending on atmospheric conditions, the fry are collected 6-8 weeks after the larvae hatch. One ha of pond surface area produces 50-250 kg of summer fry with a mean BW of 0.20 to 0.70 g. In order to harvest the summer (and fall) fry produced in earthen ponds, it is necessary to drain the water from the ponds. The fish crowd into harvesting cages placed behind the outlet box. Fry can also be harvested using floating harvesting pens placed in front of the outlet box. The fry are then transported in plastic bags with oxygenated water (30 litres water + 30 litres oxygen). From 600 to 3 000 summer fry are stocked per bag, depending on fish size, water temperature and transport time. Oxygenated transport tanks can handle from 18 000 fry/m³ (transport time 15 h, water temperature 20 °C) to 120 000/m³ (transport time 2 h, water temperature 15 °C).
The fry are stocked into a RAS that comprises circulation tanks with volumes of 1.0-3.0 m³ (tank depth >40 cm; optimum 70-100 cm). Light intensity measured at the rearing tank surface should not exceed 50 lux. The optimum temperature for rearing fry is 22 °C. The initial stocking density is 5-8 fry/litre (1.5-3.0 kg/m³). The fish are fed commercial salmonid feeds with a high protein content (>50 percent) and a fat content of 12-18 percent. In the initial phase of rearing (adaptation period to new feed) the particle size of the feed is 0.4-0.7 mm. Feed is provided ad libitum for 16-24 h per day. The daily feed ration in the first week is 15-17 percent of biomass. The adaptation period is 2 to 3 weeks. Survival during this period is 50-90 percent. Cannibalism is a factor that can limit the efficacy of this method. The fish must initially be sorted precisely. They are then sorted again after 3-4 weeks of rearing. Typically, the total length of the hatchery rearing phase is 8-12 weeks.
Intensive method (RAS)
The techniques used for rearing pike-perch larva intensely in RAS is more akin to those employed for marine than for freshwater species in both character and difficulty. There are three critical periods that impact the final success of intense rearing:
- Transformation to exogenous feeding. Larval pike-perch are tiny and after hatching their alimentary tract is a simple, anatomically undifferentiated tube. The stomach and pyloric caecae do not form until about two weeks following hatching (TL 8-13 mm; 210-273 °D). The first exogenous food must be of an adequately small size
- Inflation of swim bladder. Pike-perch are physoclistic (no connection between the alimentary canal and the swim bladder). The brief swim bladder inflation period is between 4-11 days post hatch (DPH) (84-231 °D). If the swim bladder fails to inflate during this period, normal development of the anterior segment of the larval alimentary tract renders this impossible.
- Period of intensified cannibalism. The first cannibals appear very early when the larvae reach TL 10-14 mm (256-322 °D) and intensify after the fish have grown to TL 20 mm.
Larvae are reared in 0.5-1.5 m³ circulation tanks. For the first two weeks, a spray system is used to break the surface tension of the water (this provides larvae access to atmospheric air and allows them to fill their swim bladders). Initial stocking density ranges from 20 to 50/litre and the water temperature is 20 °C. Two methods are used for the initial feeding of larval pike-perch:
- Method 1. In this method both commercial feed and Artemia nauplii are fed to the larval pike-perch during the first 14 days. During this period brine shrimp are provided at ~200-300 nauplii/fish/day, given in a minimum of 3 portions/day. After 14 days, the larvae are fed commercial feed exclusively. The granule size in the first 10-14 days of rearing is 0.1-0.3 mm, while in the third and fourth weeks it is 0.2-0.4 and 0.3-0.5 mm respectively. The feed has a high protein content (55-62 percent) and a lipid content of 10-16 percent. Feed is delivered ad libitum 24 h/day with an automatic feeder.
- Method 2. In this method larval pike-perch are fed brine shrimp exclusively for the first 14 days at a rate of ~500 nauplii/fish/day, delivered every 1-1.5 h for a minimum of 16 hours per day. After 2 weeks the larvae are trained to accept commercial feed; the period required to do this is usually 3 days. After 3-4 weeks of rearing (BW >50 mg) the fish are sorted, and the fish with inflated swim bladders (ISB) are separated from those with non-inflated swim bladders (NSB). This is carried out in an aqueous solution of sodium chloride and etomidate at a dosage of 10 g sodium and 1 ml of etomidate/litre of water. After the fish have been anaesthetized in this solution, the individuals with ISB rise to the surface, while fish with NSB sink to the bottom. After this procedure, only individuals of good biological quality (ISB individuals) are retained for further rearing.
Rearing Fingerlings
Ponds are stocked with pike-perch spawners (natural or semi-natural pike-perch reproduction), and the hatch is reared for several weeks.
Ponds (0.5 – 2.0 ha) may also be stocked with larvae (200 000-500 000/ha) obtained from hatcheries. 10-14 days before stocking larvae the ponds are filled to half their depth (40 percent volume), with the remaining water added 10 to 14 days after stocking. The ponds are fertilized with manure at the same time as they are filled with water (4-8 tonnes/ha). Rearing lasts from 6-8 weeks, survival is 15-40 percent, and production is 50-250 kg/ha of juveniles with a mean BW of 0.2-0.7 g.
Nursery
Nursery rearing in RAS systems
Juvenile pike-perch (BW 0.2-10 g) are reared in 2-5 m³ RAS tanks. Water temperature ranges from 22-24 °C. The stocking density is limited to 10/litre. The daily feed ration is 12-10 percent of biomass. During this phase, stocks are graded every 2 to 4 weeks. Light intensity is reduced from the hatchery level to 20-30 lux at the water surface. The stocking density (BW >3 g) is reduced from hatchery levels to 6 individuals/litre. The biomass of the fish observed with BW 3-10 g does not exceed 10 kg/m³. Juveniles destined for intensive on-growing are retained until they reach a minimum of 15 g BW. Typically, the nursery phase lasts 8-10 weeks.
Nursery in Ponds
Polyculture with carp
Ponds are used for less intensive carp production (500-1 000 kg/ha). The ponds are stocked with pike-perch spawners (2 males + 1-1.5 females/ha), fertilized eggs (0.5-1.0 nest/ha), hatched (2 000-10 000/ha) or summer fry (2 000–5 000/ ha). Juveniles are harvested in the fall with carp. The production is 20-25 kg/ha of fall fry with a mean BW of 10-15 g. Production is characterized by high variability in subsequent years.
Monoculture
This method is rarely used but is being developed. Ponds (0.2-2.0 ha) are stocked with 4 000-6 000/ha summer fry (BW - 0.2-0.5 g). The ponds are fertilized with manure (20 tonnes/ha) two weeks before stocking. The ponds are also stocked with fodder fish species (spawners, eggs and hatchlings of roach (Rutilus rutilus), tench (Tinca tinca) and gudgeon (Gobio gobio).
Ongrowing Techniques
Ongrowing in recirculation systems
This method is still under development; fewer than ten facilities in Europe are currently using it. Juveniles of 15-30 g are stocked. In the initial stages (BW 15-100 g), when 2-5 m³ tanks are used, the stock is maintained at 10-30 kg/m³. Larger tanks (20-30 m³) are used for the final stage, in which the fish are reared to >1 kg at a maximum stocking density of 80 kg/m³. The fish are sorted 2 or 3 times, firstly at 100-150 g, secondly at 200-250 g, and thirdly when the fish attain 500-600 g. Fish of >1 kg can be obtained after about 15-18 months of on-growing in RAS.
RAS grow-out feeds are high in protein (42-50 percent) and low in lipid (8-14 percent). In the early stages (15-80 g), feed particle size is 2.0-2.5 mm, while in the final stage (1.0-2.0 kg) it is 9.0-13.0 mm. Feed is delivered using automatic feeders at least three times per day. Typically FCR does not exceed 1.0:1.0 (BW 1.0 kg).
The thermal optimum for pike-perch growth is about 27-28 °C but fast growth rates are already noted at 23 °C. Oxygen saturation at the inflow is maintained at 100-120 percent, while at the outflow it should not fall below 50 percent. pH should be 6.5-8.2. Typical levels of total ammonia nitrogen (TAN) and nitrite (NO2-N) measured at the outflow of the rearing tanks do not exceed 0.40 mg TAN/litre and 0.15 mg NO2-N/llitre.
Ongrowing in Ponds
Pike-perch is reared as a so-called supplementary fish in polyculture with carp. A minimal stocking rate is normally used, namely 20-100 individuals/ha of fish aged 3+. After 3-4 years, the fish achieve body weights of 400-1 000 g and the production of market-sized pike-perch can be as high as 5-50 kg/ha.
Feed Supply
The pike-perch that are reared in ponds feed on zooplankton, zoobenthos, and fodder fish species.
Those reared in intensive (RAS) culture are fed salmon (Salmo salar), rainbow trout (Oncorhynchus mykiss), and sea bass (Dicentrarchus labrax) feeds produced by commercial feed manufacturers. Sinking feed is used, which is delivered with automatic band feeders either several times daily or continuously. Recently, two or three commercial feed manufacturers have begun to produce feed specifically formulated feed for pike-perch but the nutritional requirements of pike-perch are not well understood; feed formulae are currently being developed and perfected.
Harvesting Techniques
Market-sized pike-perch and carp reared in earthen grow-out ponds are caught in the fall by draining the ponds. Fish weighing 1-2 kg (usually ~1.5 kg) are produced in RAS. However, restaurants prefer fish weighing between 2-4 kg, for which they pay higher prices. Before harvesting, fish are starved for 2-3 days.
Handling and Processing
Pike-perch are susceptible to stress and sensitive to manipulations. The use of anaesthetics is recommended, for example during artificial reproduction. After manipulation, such as grading, it is recommended that the fish are bathed in a sodium chloride solution as a stress reduction measure (bath time 1 h in a 0.5-1.0 percent NaCl solution).
Slaughter yields at various stages of processing are as follows: gutted fish (84-88 percent BW); headed gutted carcass (63-66 percent BW); fillet with skin (54-57 percent BW), fillet without skin (48-51 percent). 100 g of skinned fillet of wild-caught pike-perch from natural conditions contains 215 mg of polyunsaturated fatty acids (PUFA), while the PUFA level of pike-perch cultured in RAS is 730 mg. The content of essential fatty acids - eicosapentaenoic (EPA, 20:5 n-3) and docosahexaenoic (DHA, 20:6 n-3) are 32 and 103 mg in wild-caught pike-perch and 140 and 370 mg in cultured pike-perch respectively. The ratio of n-3PUFA/n-6 PUFA ranges from 3.2 to 4.4.
Production Costs
The following contribute to the costs of producing 9-10 g fingerlings in RAS (initially stocked with 0.2-0.5 g summer fry from earthen ponds): labour 40 percent, energy 28 percent, feed 12 percent, fry 20 percent.
The cost of producing 10 g fingerlings in RAS (2009) is ~ USD 0.6/individual. Labour costs are 43 percent of the overall costs, depreciation 12 percent, and feed 7-10 percent. The way to lower costs is to increase the scale of production and to improve culture efficiency, especially survival. The cost of producing marketable pike-perch (final BW 1.5 kg) is estimated to be USD 6.2-7.0/kg.
Diseases and Control Measures
| In some cases antibiotics and other pharmaceuticals have been used in treatment but their inclusion in this table does not imply an FAO recommendation. | ||||
|---|---|---|---|---|
| Disease | Agent | Type | Syndrome | Measures |
| Bacterial infections | Aeromonas hydrophila | Bacterium | Irregular reddened skin ulcerations; anorexia | Improved water quality; NaCl (1%); antibacterial drugs in feed |
| Fungal infections | Saprolegnia spp. | Fungi | White cottony or hairy patches on eggs or fish (skin, fins, gills) | Formaldehyde; NaCl (1-2%) |
| Parasitic infections | Trichodina spp. | Ciliates | Increased mucus production; respiratory distress; swimming at water surface | Improved water quality; NaCl (1-2%) |
| Chilodonella spp. | Ciliates | Increased mucus production; rapid opercula movements; swimming at water surface | Improved water quality; NaCl (1-2%) | |
| Ichthyophthirius multifilis | Protozoan | Body (skin, fin, opercula) covered with white spores; sick fish keep rubbing against hard surface | Chloramina T | |
| Ichthyobodo necator | Protozoan | Rubbing skin on surfaces; raised scales; rapid opercula movements | Improved water quality; NaCl (1-2%) | |
| Gyrodactylus spp. | Monogean trematodes | White patches on gills & opercula | Improved water quality; Chloramina T; NaCl (1-2%) | |
Suppliers of pathology expertise
Government and scientific institutions provide services to aid in diagnosing and treating fish diseases. Assistance can be obtained by contacting local institutions responsible for providing veterinary supervision for fish health in aquaculture facilities.
Statistics
Production Statistics
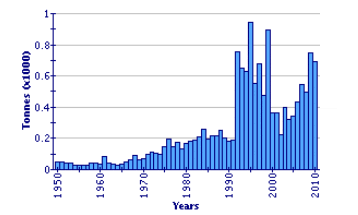
In 2009, annual aquaculture production of pike-perch exceeded 100 tonnes in only three countries - Denmark, Tunisia and Ukraine. Total pike-perch production in aquaculture (653 tonnes) in 2009 was less than 5 percent of the level caught in open waters (14 739 tonnes).
Global Aquaculture Production of Sander Lucioperca
(FAO Fishery Statistic)
Market and Trade
The pike-perch available on the market are usually caught by fishers working open waters. Kazakhstan, the Russian Federation, Finland, and Turkey made the largest catches of this species in 2009. Kazakhstan, the Russian Federation and Finland were the largest exporters. In 2008, total catches were about 20 000 tonnes, of which 9 811 tonnes were caught in Kazakhstan. However, the total catch reported in 2009 was 14 739 tonnes, of which 4 099 tonnes came from Kazakhstan and 3 011 tonnes from the Russian Federation. As is evidenced by these data, the output of capture fisheries of this species is characterized by significant fluctuations, and in recent years there has been a decreasing trend. The main importers of pike-perch include countries of Western Europe, such as Germany and France.
Thanks to its low fat content (usually 1-2 percent) and highly assimilable protein, pike-perch meat is highly valued by dieticians. Pike-perch is usually sold frozen as gutted whole fish, fillets with skin or skinned fillets. The fillets are usually sold in the following weight categories: 120-170 g, 170-230 g, 230-300 g, 500-800 g, >800 g. This species is less frequently sold fresh, e.g. whole fish, whole gutted fish, fillets with skin and skinned fillets. There are two types sold: pike-perch D (>1.0 kg) and pike-perch S (TL >45 cm, BW
Wholesale prices for pike-perch fluctuate significantly but usually range from USD 5.6-12.5/kg (whole fish) with a mean of about ~USD 8.3/kg. In some countries, such as Germany and France, prices can be as high as USD 22.2/kg.
Status and Trends
Recently, this species has been the subject of intense scientific study in both Central (Czech Republic, Hungary, Poland) and Western (Belgium, Finland, France, Germany) Europe. Research is focused on developing methods for intense pike-perch aquaculture production, mainly in RAS. Methods for artificial reproduction, including out-of-season spawning, are being refined and improved. One area of research concerns the use of cultured spawners held in RAS for reproduction, and focus is being placed on issues such as improving the quality of sex products obtained from these spawners. One of the bottlenecks in this field remains the low effectiveness and high costs of rearing larval pike-perch in RAS. Investigations conducted on this issue have included both feeding and environmental experiments. The nutritional requirements of juvenile pike-perch (quality and quantity of feed) have been largely identified.
The expansion of pike-perch culture depends on the development of culture in RAS; little or no expansion in the farming of pike-perch in ponds is anticipated. This is supported by increasing consumer demand, concomitant with decreasing catches of this species in open waters. One of the limiting factors is the possibility of producing sufficient quantities of juveniles. It is essential to improve methods for artificial reproduction and rearing larval pike-perch in RAS. Research addressing a variety of aspects related to pike-perch culture is required to:
- Develop disease prevention and treatment strategies.
- Perform genetic and selection tests.
- Determine the effects of domesticating this species.
Priority should be given to market studies aimed at identifying the needs and preferences of consumers. Good results have been obtained by consortia of researchers and entrepreneurs. One example is the project COOP 2005 – Luciopercimprove, which is financed by the European Commission.
Main Issues
Producing spawning material and commercial fish in earthen ponds, generally in polyculture with carp, is considered to be safe for the environment. This type of culture is extensive and is carried out primarily in countries where pike-perch is indigenous. Pike-perch pond culture can potentially have a negative impact on the indigenous fish population when it conducted in countries that lie outside the natural area of occurrence of this species (e.g. Tunisia).
Culturing pike-perch in RAS does not have a significant negative impact on the environment because it is conducted in the isolated environment of facilities that are mostly supplied with water from wells. The risk of fish escapes is eliminated, as are those of disease transmission. Cultivating and maintaining broodstocks in RAS frees this type of pike-perch production from the necessity of catching spawners in the wild.
Responsible Aquaculture Practices
Pike-perch aquaculture should meet the guidelines set forth in Article 9 of the FAO Code of Conduct for Responsible Fisheries, which refers primarily to the procedures and practices associated with monitoring fish health, prevention of the spread of disease, ensuring products of high nutritional value, and maintaining genetic diversity.
References
Bibliography
Berka, R. 1986. The transport of live fish. A review. EIFAC Technical Paper No. 48. FAO, Rome.
Craig, J.F. 2000. Percid fishes, systematics, ecology and exploitation. Blackwell Sciences, Oxford, UK. 352 pp.
Demska-Zak??, K. && Zak??, Z. 2002. Controlled spawning of pikeperch, Stizostedion lucioperca (L.) in lake cages. Czech Journal of Animal Science, 47:230-238.
Demska-Zak??, K., Zak??, Z. && Roszuk, J. 2005. The use of tannic acid to remove adhesiveness from pikeperch, Sander lucioperca, eggs. Aquaculture Research, 36:1458-1464.
Horváth, L., Tamás, G. && Tölg, I. 1984. Special methods in pond fish husbandry. Halver Corporation, Seattle, USA. 148 pp.
Jankowska, B., Zak??, Z., ?mijewski, T. && Szczepkowski, M. 2003. A comparison of selected quality features of the tissue and slaughter yield of wild and cultivated pikeperch Sander lucioperca (L.). European Food Research and Technology, 217:401-405.
Kestemont, P., Xu, X.L., Hamza, N., Maboudou, J. && Toko, I.I. 2007. Effect of weaning age and diet on pikeperch larviculture. Aquaculture, 264:197-204.
Kowalska, A., Zak??, Z., Jankowska, B. && Siwicki, A. 2010. Impact of diets with vegetable oils on the growth, histological structure of internal organs, biochemical blood parameters, and proximate composition of pikeperch Sander lucioperca (L.). Aquaculture, 301:69–71.
Lappalainen, J., Dörner, H. && Wysujack, K. 2003. Reproduction biology of pikeperch (Sander lucioperca (L.)) – a review. Ecology of Freshwater Fish, 12:95-106.
Molnár, T., Szabó, A., Szabó, G., Szabó, C. && Hancz, C. 2006. Effect of different dietary fat content and fat type on the growth and body composition of intensively reared pikeperch Sander lucioperca (L.). Aquaculture Nutrition, 12:173-182.
Müller-Belecke, A. && Zienert, S. 2008. Out-of-season spawning of pike perch (Sander lucioperca L.) without the need for hormonal treatments. Aquaculture Research, 39:279-1285.
Nyina-Wamwiza, L., Xu, X.L., Blanchard, G. && Kestemont, P. 2005. Effect of dietary protein, lipid and carbohydrate ratio on growth, feed efficiency and body composition of pikeperch Sander lucioperca fingerlings. Aquaculture Research, 36:486-492.
Rónyai, A. 2007. Induced out-of-season and seasonal tank spawning and stripping of pike perch (Sander lucioperca L.). Aquaculture Research, 38:1144-1151.
Ruuhijärvi, J. && Hyvärinen, P. 1996. The status of pikeperch in Finland. Journal of Applied Ichthyology, 12:185-188.
Schlumberger, O. && Proteau, J.P. 1996. Reproduction of pikeperch (Stizostedion lucioperca) in captivity. Journal of Applied Ichthyology, 12:149-152.
Schulz, C., Böhm, M., Wirth, M. && Rennert, B. 2007. Effect of dietary protein on growth, feed conversion, body composition and survival of pike perch fingerlings. Aquaculture Nutrition, 13:373-380.
Schulz, C., Huber, M., Ogunji, J. & Rennert, B. 2008. Effects of varying dietary protein to lipid ratios on growth performance and body composition of juvenile pike perch (Sander lucioperca). Aquaculture Nutrition, 14:166-173.
Siwicki, A.K., Zak??, Z., Fuller, J.C. Jr., Nissen, S., Trapkowska, S., G??bski, E., Kowalska, A., Kazu?, K. & Terech-Majewska, E. 2006. Influence of ?-hydroxy-?-methylbutyrate on nonspecific humoral defence mechanisms and protection against furunculosis in pikeperch (Sander lucioperca). Aquaculture Research, 37:127-131.
Steffens, W., Geldhauser, F., Gerstner, P. & Hilge, V. 1996. German experiences in the propagation and rearing of fingerling pikeperch (Stizostedion lucioperca). Annales Zoologici Fennici, 33:627-634.
Szkudlarek, M. & Zak??, Z. 2007. Effect of stocking density on survival and growth performance of pikeperch, Sander lucioperca (L.), larvae under controlled conditions. Aquaculture International, 15:67-81.
Verreth, J. & Kleyn, K. 1987. The effect of biomanipulation of the zooplankton on growth, feeding and survival of pikeperch (Stizostedion lucioperca) in nursing ponds. Journal of Applied Ichthyology, 3:13 - 23.
Wang, N., Teletchea, F., Kestemont, P., Milla, S. & Fontaine, P. 2010. Photothermal control of the reproductive cycle in temperate fishes. Reviews in Aquaculture, 2:209-222.
Wang, N., Xu, X. & Kestemont, P. 2009. Effect of temperature and feeding frequency on growth performances, feed efficiency and body composition of pikeperch juveniles (Sander lucioperca). Aquaculture, 289:70-73.
Zak??, Z. 1999. The effect of body size and water temperature on the results of intensive rearing of pike-perch, Stizostedion lucioperca (L.) fry under controlled conditions. Archives of Polish Fisheries, 7:187-199.
Zak??, Z. 2007. Out-of-season spawning of cultured pikeperch (Sander lucioperca (L.)). Aquaculture Research, 38:1419-1427.
Zak??, Z. & Demska-Zak??, K. 2009. Controlled reproduction of pikeperch Sander lucioperca (L.): a review. Archives of Polish Fisheries, 17:153-170.
Zak??, Z., Kowalska, A., Demska-Zak??, K., Jeney, G. & Jeney, Z. 2008. Effect of two medicinal herbs (Astragalus radix and Lonicera japonica) on growth performance and body composition of juvenile pikeperch [Sander lucioperca (L.)]. Aquaculture Research, 39:1149-1160.
Zak??, Z., Przyby?, A., Wo?niak, M., Szczepkowski, M. & Mazurkiewicz, J. 2004. Growth performance of juvenile pikeperch, Sander lucioperca (L.) fed graded levels of dietary lipids. Czech Journal of Animal Science, 49:156-163.
Zak??, Z. & Szczepkowski, M. 2004. Induction of out-of-season spawning of pikeperch, Sander lucioperca (L.). Aquaculture International, 12:11-18.
October 2012




