Mycotoxins are toxic chemicals produced by certain species of molds usually belonging to the Aspergillus, Penicillium or Fusarium genera. The importance of mycotoxins to aquaculture and animal agriculture first became apparent during the early 1960s with outbreaks of aflatoxicosis in young turkeys in the United Kingdom and hatchery-reared rainbow trout (Onchorynchus mykiss) in the United States. In both cases the origin of aflatoxicosis was aflatoxin-contaminated feed (peanut meal for turkeys and cottonseed meal for trout). Other mycotoxins described since then include ochratoxin A, deoxynivalenol, T-2 toxin, zearalenone, moniliformin, cyclopiazonic acid and fumonisin.
Aflatoxin is prevalent at certain times in corn grown in the southeastern U.S. and much research has been done on ways to mitigate its effects on corn crops and, subsequently, on animal agriculture. Plant breeders are seeking improved corn varieties that are more resistant to invasion by Aspergillus flavus, the mold that produces aflatoxin. The fungus is most problematic during hot, droughty summers, especially when developing corn kernels are damaged by insects. Aflatoxin contamination of corn can be reduced by planting resistant varieties, by irrigating to supplement rainfall, and by applying Bt (Bacillus thuringiensis) technology to control insects. Of course, failure to provide adequate storage facilities for corn and other feedstuffs can increase the levels of aflatoxin and other types of mycotoxin contamination in field crops infected with a toxigenic fungal organism. Cottonseed meal, commonly used in feed formulations for warm-water fish such as catfish, also can be contaminated with aflatoxins. Aflatoxins in feeds or feed ingredients are usually a mixture of four aflatoxins with only slightly different chemical structures. The most prevalent and most toxic to animals is AFB1. The other forms are AFB2, AFG1 and AFG2; they are included in the term “total aflatoxins.”
The term “fumonisins” includes several mycotoxins that cause the same biochemical disruption of sphingolipid metabolism. FB1 is the most toxic and most prevalent, constituting about 75 percent of the total fumonisin mixture in contaminated corn.
Some molds that can produce mycotoxins, such as certain Fusarium species, are more active during periods of prolonged moisture from excessive rainfall, as was experienced during the late summer and fall of 2009 over widespread areas of the southeastern and midwestern U.S.
Mycotoxins Produced by Aspergillus or Penicillium Genera
While rainbow trout are very sensitive to the presence of aflatoxin in their diets, with as little as 0.4 ppb (?g/kg of diet) dietary aflatoxin producing heptocellular carcinoma (HCC) in 14 percent of trout over a period of 15 months, warmwater fish do not appear to be as sensitive to dietary aflatoxin. In an aquarium study, channel catfish (Ictalurus punctatus) fed diets containing up to 275 ppb total aflatoxins from moldy corn for 12 weeks showed no reductions in weight gain or survival (more than 97 percent of fish survived for all dietary treatments, including the controls). In a pond experiment, catfish fed a practical diet containing 50 percent moldy corn and at least 88 ppb aflatoxin for 130 days showed no reductions in pond productivity, feed efficiency, or hematocrit values in comparison to catfish fed diets containing 50 percent clean corn and 1 ppb aflatoxin. In a recent study, channel catfish were fed practical diets that contained up to 135 ppb aflatoxin from moldy corn for 10 weeks and subsequently challenged with the catfish pathogen Edwardsiella ictaluri, which causes enteric septicemia of catfish (ESC). At 21 days post-challenge, these fish did not have higher mortality than catfish fed the control diet (0 ppb aflatoxin). Channel catfish appear to be able to detoxify dietary aflatoxin. Tilapia (Oreochromis nilotica) did have lower weight gains, poorer feed conversion (FCR) values, and lower hematocrit values when fed diets containing 2,500 ppb or more aflatoxin. A diet with 250 ppb aflatoxin did not produce these responses. Other research showed that tilapia had reduced growth rates when fed a diet with 1,880 ppb aflatoxin for 25 days, but not when fed a diet with 940 ppb aflatoxin. Therefore, both channel catfish and tilapia appear to be much less vulnerable to aflatoxin than rainbow trout.
Ochratoxin A (OA) is a mycotoxin produced by molds of the Aspergillus or Penicillium genera. It is more prevalent in cooler climates such as Canada and the states that border Canada. Ochratoxin A can cause kidney damage in livestock. In a study of the effects of dietary OA on channel catfish, fish fed 4 ppm (mg/kg of diet) OA in a practical diet gained less weight than control fish and experienced obliteration of the exocrine pancreatic tissue, which is associated with the hepatic portal vein. These catfish had no lesions on the renal tissue of the posterior kidney. Channel catfish that consumed practical diets containing 2 or 4 ppm OA and were subsequently challenged with the pathogenic bacteria Edwardsiella ictaluri had greater mortality than control catfish.
Ochratoxin A is an important mycotoxin not only because of its negative effects on aquaculture production, but also because it contaminates the edible tissues of fish and other animals that consume it; therefore, it can enter the human food chain and damage the renal systems of people who eat contaminated products. Because of the prevalence of OA in cereal grains used to feed livestock in certain regions of the world, OA is considered to be the cause of Balkan endemic nephropathy in humans who have consumed foods contaminated with OA. Research conducted at Auburn University showed that OA in diets of channel catfish accumulated in liver and muscle tissue slowly over time, but was also slow to clear from these tissues after withdrawal of the OA-contaminated diets.
Another Aspergillus mycotoxin is cyclopiazonic acid (CPA). Studies have shown that in channel catfish it reduces weight gain even more than similar dietary levels of aflatoxin. Concerns about co-contamination of fish feeds by aflatoxin and CPA are justifiable because both mycotoxins can be produced by A. flavus. The effects of these two mycotoxins being present together in the diets of fish have not been evaluated.
Fusarium Mycotoxins
Fumonisins, especially fumonisin B1 (FB1), are found in corn grain that is a major component of aquacultural feeds for warmwater fish. The fungal organism that produces fumonisin is Fusarium vertilloides (formerly F. moniliforme). It infects the corn plant from the soil, primarily through the root system, in a manner similar to an endophyte. Airborne spores also can infect the developing silks of the corn ear. Most corn grain probably contains at least some low level,
Deoxynivalenol (DON) or vomitoxin is a mycotoxin formed by Fusarium graminearum, which infects corn, wheat and other small grains. The toxin gets its alternative name, vomitoxin, from the fact that feeds containing >4 ppm DON usually cause vomiting in swine and chickens. Fish are not as sensitive to DON as swine or chickens. Catfish had no detrimental response to purified DON up to dietary levels of 10 ppm, but diets containing 15 and 17.5 ppm DON caused reduced growth (without vomiting). Trout fed 20 ppm DON did not vomit, but refused feed.
Another mycotoxin associated with Fusarium mold contamination of small grains is T-2 toxin. Diets containing 0.625 ppm T-2 toxin depressed weight gain, while higher levels of T-2 toxin (1.25, 2.5 and 5.0 ppm) also lowered hematocrit values. Feeding diets containing 1.0 or 2.0 ppm for only 6 weeks caused 84.1 percent and 99.3 percent mortality, respectively, after exposing the catfish to E. ictaluri in an experimental challenge.
Moniliformin is a mycotoxin produced by Fusarium proliferatum, which commonly infects corn plants. Research trials at Auburn University showed that juvenile channel catfish diets containing moniliformin at 20, 40, 60 and 120 ppm of diet significantly lowered weight gains compared to the control catfish. Moniliformin disrupts the intermediary metabolism of the tricarboxylic acid (TCA) cycle at the conversion of pyruvate to acetyl-CoA, the starting intermediate for the TCA cycle. A test for moniliformin exposure is to evaluate serum pyruvate levels; exposure to the mycotoxin causes elevated pyruvate levels. Because the molds that produce fumonisin and moniliformin prefer the same substrate, these two mycotoxins can contaminate the same lots of corn. The research described above showed that co-contamination by these two mycotoxins could produce greater toxicity, as evidenced by lower weight gains, than caused by feeding either mycotoxin alone.
Zearalenone is a Fusarium mycotoxin that has potent estrogenic effects on certain livestock animals. Feed concentrations of zearalenone as low as 1 to 4 ppm can cause transient to permanent reproductive damage in breeding swine, depending on the age of the animals; older animals are more susceptible than younger animals. Zearalenone appears to compete with endogenous estrogenic hormones for estrogenic receptors. This mycotoxin can contaminate grain crops—including corn, wheat and rice—that have been exposed to a mold organism such as F. graminearum. Zearalenone production is enhanced by cool, humid weather and delayed harvest. The effect of zearalenone on fish has not been evaluated, but since it interferes with reproduction in many animals, it should be tested on both juvenile catfish and broodstock that are approaching the spawning season.
General Considerations
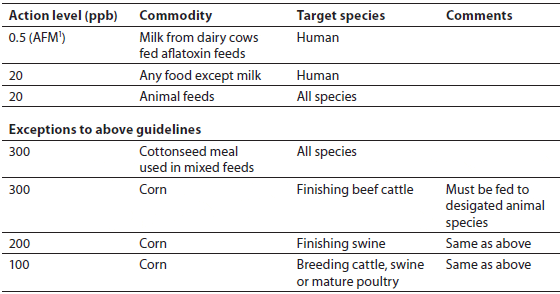
Obviously, mycotoxins are a concern for agricultural producers who grow cereal grains and oil seed crops. They also should be of concern to producers who feed these plant-derived feedstuffs to animals produced for human consumption. A testing program to detect mycotoxins should be implemented by grain elevators, storage facilities and feedmills, especially when growing conditions are likely to increase mold damage. At this time, the only mycotoxin controlled by the federal government is aflatoxin. The U.S. Food and Drug Administration (FDA) has imposed a 20 ppb upper limit (action level) for aflatoxin in foods and most animal feeds and feed ingredients. Exceptions have been made for corn and cottonseed meal that contain higher levels of total aflatoxins, as outlined in Table 1. Feed containing corn with higher levels (100 to 300 ppb) of total aflatoxins must have a designated animal species to which it will be fed.
Additionally, The FDA has issued a guidance for fumonisin and an advisory for DON on the maximum concentrations of these mycotoxins in feeds and feed ingredients. The guidance suggests that the concentration of total fumonisins in corn (representing no more 50 percent of the finished feed) used in the manufacture of catfish feeds be no more than 20 ppm, and that concentrations in finished catfish feeds not exceed 10 ppm. The advisory for DON sets a limit of 5 ppm for wheat, wheat by-products and other small grains used in fish feeds, and an upper limit in finished fish feeds of 2 ppm. Based on research conducted with these two mycotoxins, adhering to these limits should prevent problems in warmwater fish or rainbow trout.
Moldy feeds and feed ingredients that contain a known mycotoxin may also contain unknown chemical substances that have been elaborated by mold organisms. These chemical substances can be toxic to target animals and/or may augment the toxicity of the known mycotoxin. For example, fusaric acid is another toxin produced by many molds of the Fusarium genus. Fusaric acid is usually classified as a phytotoxin with limited toxicity towards agricultural animals. Even though the toxicity of fusaric acid has not been tested on fish, there is reason to be concerned about the combined effects of fusaric acid and fumonisin or DON, with which it may occur.
The effects of mycotoxins on the immune systems of fishes have been examined. Mycotoxins usually seem to impair immune responses, although (in the example cited previously) this was not the case in channel catfish fed aflatoxin diets and then challenged by Edwardsiella ictaluri. Channel catfish seem to be able to detoxify aflatoxin readily. However, the responses of juvenile channel catfish fed diets containing T-2 toxin or OA were completely different. Both mycotoxins caused high catfish mortality during a bacterial challenge with E. ictaluri. There was 99.3 percent mortality in catfish fed 2.0 ppm T-2 toxin, as compared to 68.3 percent mortality in catfish fed the control diet. Feeding a diet containing 4.0 ppm OA resulted in 80.5 percent mortality. Feeding a diet containing 80 ppm of fumonisin (FB1) increased mortality in year-2 channel catfish when challenged with E. ictaluri, as compared to the control catfish. The results of these controlled experiments suggest that exposing farmed fish to moldy feed containing mycotoxins can increase mortality during bacterial disease outbreaks.
US FDA Guidelines for Acceptable Levels of Total Aflatoxins in Foods and Animal Feeds.
Storage of Aquaculture Feeds
The importance of storing finished fish feeds properly cannot be emphasized too strongly. Feeds contaminated with toxigenic mold spores may produce mycotoxins when exposed to high moisture conditions. Condensation can form on the insides of metal storage bins during seasonal temperature changes in fall and winter. This is one good reason to empty and clean all storage bins of fish feed, unless they are in continuous use during the fall and winter months, as a precaution against the development of mold growth. When the moisture level is higher than 12 percent, mold can grow and possibly produce mycotoxins. Fish feeds stored in bags or sacks also must be protected from moisture. Bags should be stored in a building with a sound roof and solid sides to prevent their exposure to rain.
Stored feed that is found to be moldy should be tested for the presence of mycotoxins before it is fed to fish. There are many simple tests for commonly found mycotoxins. Test kits contain all the chemicals and containers needed to complete the test. Tests usually provide a yes or no answer as to the presence of the mycotoxin of interest, rather than its concentration in the feed. Therefore, this type of testing should be considered a screening for the presence of mycotoxins. If a mycotoxin is detected, further testing should be conducted to determine its concentration in the feed.
Usually there are warnings posted in the electronic media when current conditions are favorable for the production of certain mycotoxins on animal feed ingredients. This information can be used to reduce the number of mycotoxins that have to be evaluated. Commercial or state testing laboratories can test for the presence of mycotoxins in aquaculture feeds. These services usually provide information about the mycotoxins present and their concentrations. It is important to know the concentration at which the mycotoxin of interest is toxic, based of scientific evaluation of that mycotoxin for the intended species and age of fish.
Mitigation of Mycotoxins in Fish Feeds
There are adsorbents that bind feedborne mycotoxins to prevent them from being absorbed by fish after consumption. These binders fall into two main classes: 1) hydrated sodium calcium aluminosilicate (HSCAS) clays and 2) modified fractions of the single-cell yeast organism Sacchromyces cerevisiae, or common bakers’ yeast. The clays seem to work well with aflatoxins, but are less effective with other mycotoxins. The yeast preparations appear to be effective on a broader range of mycotoxins. Neither type of binder has been extensively evaluated in fish feeds. Agents that are purported to bind mycotoxins should be tested on the mycotoxin of interest to be certain that effective binding occurs and that the binder is safe for the intended species of fish.
The cooker-extrusion process of feed manufacturing, which applies heat, reduces the level of aflatoxins in channel catfish feeds. In catfish pond experiments, preparing floating feeds containing aflatoxin-contaminated corn by cooker-extrusion technology reduced the level of aflatoxin by more than 60 percent. Earlier research showed that heat chemically transforms aflatoxin into aflatoxin breakdown products. There is little chemical breakdown of fumonisin, OA, DON and T-2 toxin as a result of applying heat during cooking or feed manufacturing.
The effect of moldy feeds, and the mycotoxins and other chemical substances they produce, on the growth and health of cultured fish is not well understood. In fact, the identity of the many chemical substances feed-associated molds produce may not be complete. Because so little is known, it is prudent to prevent fish feeds from becoming moldy and to refuse to purchase feeds and feed ingredients that are moldy, even if they are offered at a discount. If stored fish feed has become moldy, do not use it until it has been evaluated for mycotoxin contamination.
Additional Reading
Council for Agricultural Science and Technology. 2003. Mycotoxins: risks in plant, animal, and human systems. Report 139. Ames, Iowa.
Michael H. Henry. 2006. Mycotoxins in feeds: CVM’s Perspective. Division of Animal Feeds, Center for Veterinary Medicine, Food and Drug Administration, Washington, D.C. (slide presentation available at FDA website)
D. E. Diaz, editor. 2005. The Mycotoxin Blue Book. Nottingham, U.K.: Nottingham University Press.
H. K. Abbas, editor. 2005. Aflatoxin and Food Safety. Boca Raton, Florida:Taylor and Francis Group.