Mycobacterial diseases of fish are common, particularly in intensive aquaculture systems and display aquaria. These diseases are collectively referred to as “atypical mycobacteriosis” or simply “mycobacteriosis.” The term “fish tuberculosis” has been used in the past to refer to this group of diseases, but the term is not appropriate and should not be used. Tuberculosis is a very important disease of humans and mammals, but fish do not get tuberculosis.
All fish are susceptible to mycobacteriosis, though some species seem to be at greater risk than others. The disease has been reported in a broad range of fish species from freshwater, marine and brackish water environments. In freshwater systems, the centrarchids, especially striped bass and their hybrids, seem especially susceptible. In mariculture, mycobacteriosis has been reported in sea bass, turbot, Florida pompano and others. In marine aquaria, the sygnathids (members of the sea horse family) are notoriously susceptible.
Mycobacteriosis was formerly a problem in the salmon industry, but since the feeding of fresh fish offal (waste products from slaughtered fish) was discontinued and modern processed diets were developed, the problem has largely disappeared. The susceptibility of aquarium fish may be related, at least in part, to their longevity. Fish in the families Anabantidae (bettas and gouramis), Characidae (tetras), Cyprinidae (barbs, danios, koi and goldfish), and some members of the Cichlidae (including freshwater angelfish) may be more prone to the infection.
This disease is also of concern in recirculating systems and once established can be difficult to eradicate. High organic loads, water quality characteristics common in intensive systems, and very crowded populations can all exacerbate the infection. Mycobacteriosis is an Centerimportant infectious disease in zebrafish colonies so it is important to avoid introducing infected fish into valuable research colonies.
In addition to causing disease in cultured fish, atypical mycobacteriosis is also a concern in zoological collections. It is the most important disease of sea dragons and other members of the sea horse family. It has also been reported in cultured amphibians and reptiles, as well as in other aquatic animals from a broad range of taxa.
Though rare in mammals, mycobacteria sometimes cause localized and systemic disease in captive marine mammals, including manatees, pinnipeds (seals or sea lions), and cetaceans (dolphins and small toothed whales). The disease is most often seen in animals reared under suboptimal conditions, including those that may be stressed or immunocompromised. Mycobacterial disease may also be associated with long-term use of corticosteroids in some individuals. Mycobacterial diseases are zoonotic, which means that they can affect humans who come in contact with infected fish or environments.
Mycobacterium causes a chronic disease, usually characterized by wasting. It should be suspected when fish are in poor condition and also have scale loss, skin ulcers, or a history of reproductive problems. Occasionally, deep hemorrhagic skin lesions will be seen in addition to the more common superficial lesions. Because the disease can masquerade as a number of other conditions, samples should be taken from any fish showing signs of chronic disease or reproductive problems and analyzed with bench-top acid-fast staining.
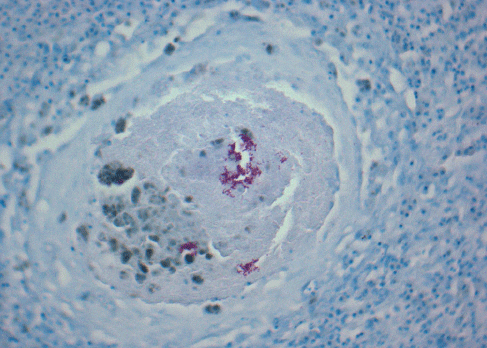
The typical lesion caused by mycobacteriosis is granulomatous inflammation. Granulomas (Fig. 1) signify the body’s effort to isolate an irritant or foreign body of some sort. Typically, granulomas are recognized by the appearance of a wall of tissue around the area where the bacteria are active. Granulomas are often seen during microscopic examination of infected tissues, but in advanced cases they may be visible to the unaided eye (Fig. 2). Although considered typical of the disease, granulomas do not develop in all cases.

Vaccines are not currently available, though they may be developed in the future. There is no effective treatment for infected fish, so prevention through the use of quarantine and disinfection protocols is critically important. Populations of fish that harbor the infection are most often euthanized and the system they were housed in disinfected with appropriate agents. Currently, there are no non-lethal tests for screening fish for mycobacterial diseases.

Atypical Mycobacteriosis and Associated Diseases
Organisms Associated with Mycobacteriosis in Fish
Historically, three species of Mycobacterium spp. have been most commonly reported in fish. These are M. marinum, M. fortuitum, and M. chelonea. The names of these organisms are confusing because some reports may have called the organisms by new names, as described below, or names may been have been changed over time. To minimize confusion, a brief effort has been made to identify some of these changes and provide the reader with current nomenclature.
The first report of mycobacterium was in 1897, from a diseased marine aquarium fish. At that time, the organism was named M. piscium. Although unproven, the organism originally referred to as M. piscium is believed to be the same thing as the one called M. marinum today. Subsequent reports of new isolates called M. anabanti and M. platypoecilus have been shown to also be M. marinum. Another common isolate today is M. fortuitum, which was initially isolated from a neon tetra in the 1950s. Since then, organisms originally identified as M. ranae and M. salmoniphilum have been shown to be M. fortuitum. Mycobacterium chelonae has been associated with disease in salmonids raised in cold water conditions. Recently, the organism has been subdivided into two subspecies, M. chelonae and M. abscessus, with M. abscessus being an important pathogen of the Japanese medaka fish. A recent review of current nomenclature and the association of different species of mycobacterium with diseases in specific groups of fish was provided by Noga (2010).
An important new isolate of mycobacterium that causes disease in wild striped bass (Morone saxatilis) in the Chesapeake Bay was reported in 2001. This new isolate was similar to M. marinum and M. ulcerans, but was not identical to either. This group of organisms are genetically similar to M. tuberculosis, the causative agent of tuberculosis in humans. It is important to emphasize, however, that genetic similarity does not mean that fish get tuberculosis. One of these organisms, Mycobacterium ulcerans, does cause disease in humans, but it does not cause tuberculosis. The presence of this organism in tilapia has been reported, but the finding was considered “unconfirmed” by other researchers, so the significance of M. ulcerans to fish, or to people handling fish, is not well understood at this time.
Many other species of mycobacterium have been isolated from fish, including M. smegmatis, M. neonarum, M. simiae, M. scrofulaceum, M. poriferae, and an M. triplexlike organism. Although developing a clinical, or presumptive, diagnosis of mycobacterium is fairly straight-forward, identifying the organism to species requires the use of culture and molecular techniques. In some cases this can be important, as discussed below. But often it may not be necessary to identify the organism to species in order to make good management decisions when an outbreak occurs.
Researchers have compared the ability of several different mycobacterium isolates to cause disease in zebrafish. Three isolates of M. marinum were tested, two from clinical cases (i.e., the organism was isolated from diseased fish) and a third from the American Type Tissue Collection (ATTC). Strains of M. chelonae, M. abscessus, and M. peregrinum, all of which had been isolated from zebrafish, also were tested. Of these isolates, only the two strains of M. marinum taken from active outbreaks of mycobacteriosis in zebrafish were considered highly pathogenic. These two strains of M. marinum caused 90 to 100 percent mortality of injected zebrafish within 21 days, with the first mortalities observed about day 7. This work demonstrated that there are measureable differences in pathogenicity (the ability to cause disease) between different strains of these organisms and that some of these differences may be important enough to warrant identifying isolates to the species level.
In addition to being present in infected fish, mycobacterial organisms also live in filter media and biofilms. One study showed that despite the presence of diverse flora of mycobacterial species in the environment, only one organism, M. marinum, was found in fish tissue. Molecular tests can be used to identify isolates taken from fish and match these genetically to isolates taken from environmental sources.
Environmental Conditions that Favor Atypical Mycobacterium
Mycobacterial organisms thrive under certain environmental conditions, including warm water temperatures, low dissolved oxygen levels, acidic pH, high soluble zinc, high fulvic acid, and high humic acid. Many of these conditions—especially the low dissolved oxygen, low pH, and an organically rich environment—are present in intensive aquaculture systems. An important part of preventing disease is decreasing the exposure threshold of host fish. If the environment favors growth of the organism, there will be greater numbers available to infect fish housed in the system. This can significantly increase the risk of infection.
Although these organisms are common in aquaculture environments, there is evidence that disease in fish may be associated with certain strains of mycobacterium rather than with all the environmental isolates present. Poor husbandry, chronic stress, or anything else that impairs the immune function of the fish will increase the likelihood that infection will develop. Because some isolates are more pathogenic than others, eliminating these specific isolates from a system is critical to stopping disease progression and mortality. Much of this will be done by carefully culling sick fish or completely depopulating, as is usually recommended. Less pathogenic (or environmental) isolates of mycobacterium can be controlled by creating a more hostile environment for the organism. This decreases the risk of clinical disease and mortality even though low levels of the organism remain in the environment. Specific strategies for manipulating the environment to favor the fish over the bacteria are discussed below.
Life History and Transmission
The mycobacterial agents of greatest concern to aquaculture are those that are actively causing infection in fish. Infected fish release the organism from skin and gill lesions and shed them from the gastrointestinal tract. When sick fish die and decompose, bacteria are released from internal organs. Infection can be spread when fish have direct contact with infective material or ingest infected tissue. The best example of this is from the salmon industry, which early in its development fed unpasteurized fish offal that caused a serious, industrywide problem with mycobacteriosis. Since this practice was discontinued, the disease is rarely reported in salmon hatcheries. Animals that die of mycobacteriosis must be removed from a collection or culture system so that other fish will not eat the infected carcass and spread the disease.
In addition to horizontal transmission (fish to fish), vertical transmission (mother to offspring) also occurs. Vertical transmission is well documented in live-bearing fish, but is less well understood in egg-laying species, though it is known that the organism occurs in gonadal tissue. Vertical transmission should be assumed until more information becomes available. Depopulation of mycobacterium-infected broodstock is recommended.
Potential Impact on Aquaculture Businesses
Mycobacterium is of great concern to aquaculture industries, especially to businesses that use recirculating systems. Once a population of fish is infected, the organism is magnified, creating a vicious cycle of infection, disease and mortality. Although steps may be taken to decrease the severity of infection in a group of fish, the disease cannot be reasonably treated and cannot be cured. Infected broodstock must be presumed to infect their young. Producing infected fish makes little economic sense, as fish from infected parents will likely be stunted, have chronic mortality, and pose a risk to other fish, employees, and consumers. An aquaculture system that becomes infected should be depopulated and completely disinfected.
Special Considerations for Laboratory-reared Zebrafish
Mycobacteriosis may be the most common infectious disease in zebrafish colonies. Historically, fish were exchanged between different laboratory groups and introduced to new facilities with little attention paid to quarantine or disease screening. These practices have largely been discontinued, and quarantine protocols to prevent the introduction of mycobacterium and other infectious agents are in place at many research facilities.
Mycobacterium marinum, M. fortuitum, M. chelonae, M. abscessus, and other isolates are all important pathogens of zebrafish in laboratory settings. Zebrafish infected with M. fortuitum show signs of “dropsy” (fluid accumulation in the abdomen, scales protruding from the body wall), while those infected with M. chelonae and/or M. abscessus develop skin erosion, ulceration, and granulomas within the visceral organs. Zebrafish experimentally infected with a highly pathogenic strain of M. marinum developed peritonitis and granulomas in multiple organs and had mortality rates of 30 to 100 percent. In contrast, fish experimentally infected with non-marinum mycobacteria had little mortality and only occasional granuloma formation in visceral organs. These differences in disease severity and resultant mortality emphasize the importance of identifying specific mycobacterial isolates when the disease is suspected.
To eliminate mycobacteriosis from a colony of zebrafish, the system must be depopulated and completely broken down to its component parts. All filter media and disposable materials should be discarded, including plastic tanks. Chlorine bleach can be used to break down organic material and biofilm inside pipes and in other inaccessible areas, but it is also essential to treat all surfaces with a mycobacteriocidal agent such as ethyl alcohol (50 to 70 percent) or Lysol® before restocking. Methods of disinfection are discussed below. Because there is no non-lethal method of screening live fish for mycobacterial disease, representative fish from new populations should be euthanized and screened using histology (including acid-fast stains), culture, and PCR methods. This is an important part of quarantine protocols and is discussed in greater detail below.
Special Considerations for Zoos and Aquaria
Mycobacterium is not uncommon in fish and amphibian collections in zoos and aquaria. The longevity of collection animals contributes to chronic, low-grade infections within some exhibits. In exhibits with obvious disease, poor quality specimens, and chronic mortality, depopulation and disinfection should be strongly considered. If the decision is made to maintain a mycobacterium- positive population of fish in a zoological setting, certain management strategies must be put in place. These include careful attention to sanitation in the infected system (e.g., cleaning and removal of biofilm from all surfaces and routine cleaning of substrate), using UV or ozone to decrease the number of bacteria in the water column, conscientious culling of animals that become symptomatic, and posting signs to remind employees to take precautions (such as wearing gloves) when working near the infected exhibit. Finally, there must be a policy requiring that the exhibit be managed as a “closed” population, meaning that no new animals will be added to the infected population and no live animals will be removed for housing elsewhere.
The disease is a particular problem for aquaria housing sea dragons. Research has shown that cultured mycid shrimp are sometimes contaminated and may be a source of exposure when fed to sea dragons. Early detection of the disease is accomplished by carefully examining wet mounts taken from areas of erosion or ulceration, if these are observed in resident fish. Treatment with antibiotics may slow the course of disease, but will not cure infected animals.
Also of interest to zoo and aquarium professionals is the association of an incompletely characterized mycobacterial agent with granulomatous skin lesions in green moray eels (Gymnothorax funebris). Biopsy of suspect lesions may reveal acid-fast organisms. Efforts to culture the organism have been less than rewarding. If detected, molecular testing of suspect tissue may be helpful.
Atypical mycobacteriosis has also been reported occasionally in captive marine mammals, especially when animals are housed under sub-optimal conditions. The most common symptoms are skin lesions and panniculitis (inflammation of the subcutaneous fatty tissue), but systemic disease and mortality have been reported. Longterm treatment of captive marine mammals with steroids may be a risk factor.
Diagnosing Mycobacteriosis
Mycobacteriosis is typically a chronic and progressive disease and should be suspected when there is weight loss or loss of condition, especially when accompanied by scale loss, ulcers, or non-specific hemorrhagic lesions (Fig. 3). A “dropsy-like” presentation characterized by extreme abdominal distention, fluid accumulation, and scale protrusion has been reported in zebrafish infected with M. fortuitum. There may also be a history of ineffective use of antibiotics if fish are mycobacterium-positive. Occasionally, exophthalmia will be observed in infected fish. The disease, however, can manifest itself in many different ways so there is no typical presentation.
Reproductive problems are often part of the history of a mycobacterial case. Increased mortality and reproductive problems have been reported in affected zebrafish colonies. Because of the low level and chronic nature of mortality, poor reproductive performance may be the primary complaint that leads to diagnostic investigation of these fish. Although granulomas are a classic lesion associated with mycobacteriosis, they do not always develop. Further, Mycobacteria spp. have been isolated from tissue of clinically normal fish in the absence of obvious disease or pathology.

Developing a Presumptive Diagnosis
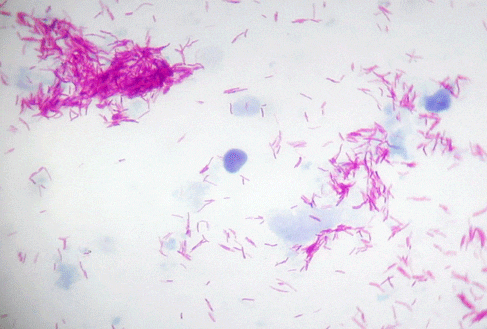
A presumptive diagnosis of mycobacteriosis is usually based on the examination of suspect fish. Any fish from a population experiencing chronic mortality, especially if accompanied by poor condition or emaciation, should be considered suspect for mycobacterium. Fish with chronic ulcers or unexplained reproductive problems also should be suspect, especially if lesions remain after appropriate antibiotic treatment. Preliminary diagnosis is usually based on the presence of granulomatous lesions, either grossly visible or microscopic. Typical granulomas have a thick capsule, walled off by epithelioid cells, and a necrotic center (Figs. 2 and 4). Acid-fast organisms are often visible within the center of the granuloma (Fig. 4). Bench-top acid-fast stains on fresh tissue are recommended to support a presumptive diagnosis of mycobacterium (Fig. 5), though extreme care should be taken when handling suspect tissue.
Observing acid-fast staining rods during histological examination would be strong support for a diagnosis of mycobacteriosis (Fig. 4). Organisms are most likely to be present either intracellularly or extracellularly within granulomas, although if there are few live bacteria in tissue the acid-fast rods may not be seen. The ability to see acid-fast rods in tissue can also be affected by processing. The decalcification process, in particular, may prevent organisms from taking up the acid-fast stain. Immunocytochemistry can be used to support a diagnosis of mycobacterium and may be more sensitive than the visualization of organisms using special stains. However, it will not identify the organism to species.
Culture Techniques for Mycobacterium spp.
Definitive diagnosis requires culturing with special media to isolate these organisms. Media used to culture mycobacterium include Petragnani, Lowenstein-Jensen, Middlebrook 7H10, and Dorset egg media. Incubating cultures at 20 to 30 °C is recommended, and 2 to 30 days will be required for preliminary growth to be visible. Culture can be quite difficult, as many organisms in this group are fastidious and are easily overgrown if contaminants are present. Mycobacterium fortuitum and M. chelonae are considered the more rapid growers, with growth being observed in about 7 days, while M. marinum may not be visible for up to 30 days. All species of mycobacterium are Gram-positive rods, non-motile, non-spore forming, and acid-fast staining. Sizes range from 1.0 to 4.0 ?m x 0.2 to 0.6 ?m.
Because these organisms can be so difficult to isolate, one culture technique that has been reported to work well will be described here. Briefly, fish to be cultured should be euthanized (MS-222—tricaine methanesulfonate—is commonly used). Five minutes after opercular movements have ceased, the external surface of the fish should be disinfected with 70 percent ethyl alcohol. Viscera should be removed using aseptic technique, and kidney, spleen and liver should be placed in 5 ml of phosphate buffered saline. These tissues are ready for submission to a diagnostic laboratory for culture and identification. Mycobacterial organisms must be handled in Biosafety Level 2 facilities, which have specific requirements for equipment and personnel. Cultures of these organisms are highly concentrated sources of infectious materials and should never be handled by untrained personnel.
Molecular Tests for Rapid Diagnosis
Because culturing these organisms is difficult and time consuming, using molecular techniques to rapidly and accurately identify them is important. Several authors have described PCR-based techniques that allow rapid diagnosis (usually 1 to 2 days) and identify organisms to the species level. These techniques have been used on tissue and blood. The ability to detect the organism in blood is promising for the development of non-lethal screening techniques that detect infected fish. Identifying the organism to species is important because it may help identify the source and potential severity of the infection. As mentioned above, pathogenicity (ability to cause disease) can vary tremendously among isolates.
Differential Diagnosis
A differential diagnosis involves considering all possible causes of an observed disease condition and distinguishing the various possibilities from each other. Because signs of mycobacterium infection are so non-specific, it is extremely important to rule out other diseases that could cause similar clinical signs.
First, there is a granulomatous disease of cichlids caused by the parasite Cryptobia iubilans that can be easily misdiagnosed as mycobacterium. Both diseases cause granulomas in infected tissue; however, granulomas caused by C. iubilans are usually observed in the gastrointestinal tract, especially the walls of the stomach. Granulomas caused by mycobacterium tend to be located in the visceral organs, especially the spleen. If a thickened stomach wall and granulomatous lesions in wet mounts of stomach tissue are seen in any cichlid, C. iubilans should be considered a likelier cause than mycobacterium. Sometimes the living flagellates can be seen in wet mounts of cryptobia-infected tissue. This would definitively distinguish the disease from mycobacteriosis. When organisms are not seen, a presumptive diagnosis of C. iubilans can be made based on affected tissue (stomach) and species (cichlid). Still, histology and possibly electron microscopy or PCR-based techniques would be needed to definitively distinguish these two diseases.
Other organisms that are easily confused with mycobacterium are Nocardia spp., which are weakly acid-fast and rod-shaped. However, Nocardia is unique in that it will be visible in tissue as branching rods. Nocardiosis is quite rare in fish, as these organisms tend to be associated with soils rather than water, but it is seen occasionally and the lesions it causes would be characterized as granulomatous. Histology will differentiate the two infections because the branching rods characteristic of nocardia should be easily seen in the infected tissues.
Finally, not all mycobacterial infections produce granulomatous lesions. A case of M. marinum in frogfish was characterized by a histiocytic inflammatory response and the complete absence of granulomas. Although granulomas were not seen, acid-fast rods were visible in almost all organ systems, with a strong predilection for macrophages. The infected fish had developed spawning problems and several females were “egg bound,” which means that they had been unable to release eggs from the abdomen. Mycobacterium infection was diagnosed only after histologic sections were stained to look for acid-fast organisms. The organism was ultimately cultured and identified as M. marinum.
Treatment Strategies
Treating Food Fish
There are no FDA-approved treatments for mycobacteriosis in cultured food fish, nor are there any unapproved products that are effective. Maintaining a population of mycobacterium-positive fish makes little sense. The fish will have chronic health problems and poor growth and feed conversion rates. Infected fish will be a constant source of infection to other fish, to employees, to processors, and to consumers. If mycobacterium is suspected, the diagnosis should be confirmed using culture and/or molecular tools and the causative agent should be identified to species. The infected system should be depopulated and all equipment disinfected with a mycobacteriocidal agent such as Lysol® or concentrated (50 to 70 percent) ethyl alcohol. The environment should be assessed and appropriate changes made to decrease the potential for future mycobacterial outbreaks. The importance of effective biosecurity in this type of situation cannot be overstated. The potential loss to a company will be far less if the infection is isolated to a single system than if it spreads throughout a production unit.
Treating Ornamental Fish
Mycobacterial infections of all fish should be considered non-treatable. Although there are some research reports of aquarium fish responding to antibiotic therapy, individual fish have not been cured of the disease. Symptoms may resolve temporarily but often reappear when antibiotics are discontinued. As described for food fish, depopulation and disinfection of all contaminated equipment is recommended. There may be rare instances when pet owners or professional aquarists may elect to maintain a population of mycobacterium-positive fish, but commercial growers of ornamental fish should always depopulate and thoroughly disinfect contaminated systems. Infected fish will spread the infection to their offspring, which will be a chronic problem for the producer and a risk to employees of the farm, the wholesale facility, and the pet store. Ultimately, it will be a risk to the consumer.
Managing Infected Populations
In the rare circumstances where maintaining a population of infected fish may be a reasonable course of action, protocols must be followed to minimize risk to humans who have contact with infected fish or equipment. Such situations are most likely to involve pet fish with significant emotional value to the owners, zoological collections, and possibly valuable research animals, although investigators should consider the impact of mycobacterium on research findings. Steps can be taken to minimize the impact of the disease over time, but because there are no screening methods for determining whether live fish are clear of the infection, such populations should be maintained as closed groups. That means no animals leave the collection for relocation with other groups of fish and no new fish are introduced to the population.
Steps that can minimize the impact of the disease include strictly maintaining optimal water quality conditions for the species, correcting environmental conditions that favor the organism, promptly removing symptomatic fish from the population (as these may shed large numbers of infectious particles), and disinfecting equipment (including using UV filters and/or ozone).
Resident fish can be temporarily removed from the infected system to allow the thorough cleaning and disinfection of all surfaces, including pipes. Gravel and filter media should be discarded and replaced. If monitoring is required, periodic euthanasia of a few representative fish for diagnostic testing will be necessary. Even if a nonlethal blood test becomes available in the future, relying on the validity of a negative test result seems unwise given the diversity of mycobacterium species.
Preventing Infection
Preventing disease is always more cost-effective and rewarding than treating it. This is especially true in the case of mycobacterium, as no vaccine or satisfactory treatment is available. Once a population of fish is infected, the most likely scenario is euthanasia of the entire group, followed by thorough cleaning and disinfection of all surfaces with a mycobacteriocidal agent.
Quarantine and Biosecurity
Biosecurity in aquaculture means that aquatic organisms are protected from the introduction of new diseases to the farm. One of the basic tenets of biosecurity is avoiding the introduction of sick animals in the first place. This is accomplished by controlling the source of new animals, testing animals before arrival at the farm and/or before introducing them to established groups, and quarantining new animals upon arrival.
Fish should be purchased only from trusted sources. Acquiring wild fish is risky; if there is a need to bring wild stock in, they should be put through a rigorous quarantine before being allowed contact with the regular population of fish on the farm. When purchasing fish, it is important to have a good relationship with the supplier. If a good rapport has been established, there may be some assurance that the supplier will be conscientious about looking for signs of mycobacterium, or other disease, before transport. Realistically, however, this is not always possible, and even careful suppliers may miss subtle signs of infection.
If a population of fish is to be tested for mycobacterium, the first step will be to euthanize several representative animals from the group. Tissues are collected during necropsy; fresh material is examined microscopically and fixed tissues are examined histologically. Special staining techniques that light up acid-fast positive organisms by staining them bright pink (Figs. 5 and 6) are very useful in determining that mycobacterial agents may be present. Staining will not allow identification of the organisms that are seen, but it does guide diagnosticians in determining whether special culture and/or molecular testing should be done. This combination of methods (necropsy, special staining of fresh and fixed tissues, culture, and molecular testing) has a good chance of detecting a positive individual in a group.
New fish should be quarantined from established populations for at least 30 days. During this time, individuals can be tested. This is very important for aquaculture businesses and zoological collections, and also should be done, when possible, to protect pets in the home aquarium or garden pond.
Once new fish are determined to be mycobacteriumfree and are introduced into established populations, routine screening should continue as part of necropsy or health management protocols. Culturists should manage the environment properly to discourage the colonization of mycobacterial organisms. This means not allowing organic material to accumulate, cleaning substrate and filter media regularly, and using ozone and/or UV sterilization to help keep mycobacterium numbers low.
Euthanasia and Depopulation
Euthanizing animals, especially if they are clinically normal and appear healthy, can be very troublesome. Yet euthanasia (for testing) and complete depopulation (to control disease outbreaks) are critical in managing this disease. Although research has shown that mycobacterial agents are not all equally pathogenic, there is also increasing evidence that organisms taken from infected fish are usually more dangerous to other fish than organisms isolated only from the environment. Sick fish produce and shed large numbers of pathogenic organisms. This is not desirable in any culture situation, and these animals need to be eliminated regardless of the broader management strategy that may be pursued.
There are many options for euthanizing fish. The important point is that animals are killed quickly and in a way that minimizes discomfort and distress. Tricaine methanesulfonate (MS-222), applied at concentrations of 1 g/L (buffered with 2 g/L of sodium bicarbonate), is often used to euthanize pet fish or fish in zoological collections. Carcasses of fish euthanized with MS-222 are not safe for human consumption! Carbon dioxide, decapitation, or electrocution are often used as euthanasia methods for fish that will become human food. “De-watering” has been used to kill large numbers of fish in a production setting, though this method is certainly less humane than some others. Circumstances and the planned disposition of animals will influence the method selected. It is also important to ensure that the fish have actually died. Ectothermic (cold-blooded) animals may continue to have beating hearts for hours after they appear to be dead.
Disinfection Protocols
Cleaning a system that is known to be infected with mycobacterium requires extra effort. The system should be thoroughly cleaned and bleached, following usual protocols, to remove organic material and biofilm that may harbor organisms. Afterward, all surfaces in the system should be disinfected with a mycobacteriocide.
Mycobacterial organisms are more resistant to disinfection protocols than most bacteria encountered in aquaculture settings. A waxy coating in the cell wall of mycobacteria gives them extra protection from many common disinfectants, including bleach. Effective mycobacteriocidal agents include Lysol® (1% benzyl-4- chlorophenol-2-phenylphenol), sodium chlorite, and ethyl alcohol at 50 or 70 percent concentrations, but not 30 percent (which requires at least 10 minutes of contact time). Chlorine bleach (sodium hypochlorite), even at concentrations as high as 50,000 mg/L, is only moderately effective in reducing the number of mycobacterial agents in the environment. Roccal® and Virkon®-S are ineffective.
Personnel should wear protective clothing during this process and prevent the material cleaned from the system from contacting any breaks in their skin (cuts or abrasions). Remember that biofilm may contain large numbers of organisms.
Zoonotic Considerations
Mycobacterial infections of fish are zoonotic, which means the organisms can cause disease in humans. These infections are relatively rare, especially when you consider how ever-present the organisms are. Early reports of atypical mycobacterial infections of humans were associated with public swimming pools, resulting in the term “swimming pool granuloma.” Mycobacterium must be considered a potential occupational hazard for workers in the aquaculture and aquarium industries.
Infections are usually associated with cleaning aquaria or with injury from contact with fish. The most common symptom in human patients is skin lesions that develop on the hands or extremities where broken skin may have come into contact with infective material. These lesions are often called “fish tank granuloma” or “fish handler’s disease.” Lesions in humans may develop from 3 weeks to 9 months after contact with infective material.
Although mycobacterial lesions in humans are typically restricted to the extremities, particularly the skin, deep lesions into musculature and tendons have been reported. Rarely, systemic disease has occurred in immunocompromised individuals.
Diagnosis in humans is accomplished by biopsy of suspect lesions. It is important that people with symptoms tell their doctors about any water or aquariumrelated activities in which they have engaged. Culturing the organism can be extremely difficult in human cases, partly because clinical laboratories tend to incubate microbial samples at 37 °C, an inhospitable temperature for these organisms. PCR-based techniques may reveal the presence of the organism even if cultures are negative. Although a number of antibiotics have been used to treat the disease in humans, there is no single protocol that has proved efficacious. In healthy individuals, superficial lesions caused by atypical mycobacteriosis should resolve; however, antibiotic therapy is recommended to prevent the infection from spreading. Corticosteroids should not be given to patients infected with mycobacteriosis.
Protecting Workers
There are specific steps that can be taken to protect workers. Any system known to be infected should be clearly labeled so that all employees are reminded of the risk when they approach the area. Employees should wear gloves when cleaning tanks or handling aquarium gravel or (dirty) filter media. Individuals with breaks in their skin (i.e., cuts or other abrasions) should have no direct contact with mycobacteria-infected areas. Skin wounds should always be covered, preferably with a bandage and water-tight gloves. Obviously, gloves should be worn when handling fish as well, especially during necropsy. Long pants or full-length wet suits and protective foot coverings should be worn when working in a tank or area with contaminated substrate or gravel. Face masks (covering the mouth and nose) should always be worn when workers are pressure washing any area. Personal hygiene should be emphasized, and alcohol-based hand sanitizers should be conspicuously located throughout the work area. Employees should be educated about the health risks associated with their jobs, and they should know to discuss these risks with their physicians as appropriate. Under no circumstances should an immunocompromised individual be allowed direct contact with mycobacteriuminfected material.
Summary
Nontuberculous mycobacteria are common organisms in the aquatic environment. Many species have been described and many have been renamed, which can make taxonomy confusing. The three species of significant concern to aquaculture and related businesses are Mycobacterium marinum, M. fortuitum, and M. chelonea. All fish should be considered susceptible to these organisms, though they may pose the greatest risk to aquarium species and fish raised under intensive conditions. The disease is common in laboratory–reared zebrafish. Members of the sea horse family are also highly susceptible to mycobacteriosis. Amphibians, reptiles, marine mammals, and even humans are susceptible to infection with these organisms, although they do not usually grow well at mammalian body temperatures.
Typical signs of mycobacterial disease in fish include weight loss or emaciation, scale loss, ulcerations or hemorrhage along the body wall, poor appetite and attitude, and often a history of reproductive problems. Some fish may appear completely normal and robust yet show evidence of infection at necropsy. The granuloma is the typical lesion attributed to mycobacterial infections, but some infected fish do not have them. Diagnosis of mycobacterial disease is presumptive when acid-fast rods are seen in the center of granulomatous lesions or in tissue, but additional testing is required to confirm the identity of the organism.
Quarantining new fish is a very important preventive measure. Currently, the only methods of detecting mycobacterium infection in fish are lethal ones. While in quarantine, a representative sample of fish from the new population should be euthanized and tissues tested during and after necropsy. Diagnostic methods include looking for granulomas in viscera and using special tissue stains to reveal the pink, acid-fast rods that indicate mycobacterium. Suspect tissue can be cultured or subjected to molecular tests to identify the specific organism. This level of testing is not practical for all situations, but careful observation of fish during the quarantine period, and careful necropsy of animals that die while in quarantine, will help detect a mycobacterium-infected population before they are released from quarantine. Mycobacterial diseases are not treatable and in most cases infected fish populations should be euthanized and their housing unit carefully cleaned and disinfected using mycobacteriocidal agents.
Mycobacterial diseases can infect humans, so workers must be protected. Individuals who are immunocompromised should not have contact with mycobacteriumpositive fish or their environment. Although the risk of disease transmission to healthy adults seems low, employees must be informed and educated about the risk so they can take appropriate precautions.




