Otherwise, biomass can be obtained from secondary and tertiary treatment of effluents. Wastewater treatment utilising photosynthetic organisms is an interesting alternative to reduce the ecological impact of domestic, industrial or aquaculture effluents. Generally,high-quality algal biomass is yielded from algal cultivation,representing an excellent source of hydrocolloids, carotenoids, and bioactive substances, which allows different industrial applications. In addition,there is currently an increasing interest for the potential of SW in human and animal nutrition.
Seaweed as ingredient in aquafeeds

Although nutritional properties of SW are not as well known as are those of land plantbased ingredients, their chemical composition may be characterised by low content in lipids, moderate in protein, but rich in non-starch polysaccharides, minerals and vitamins. Lipid contents range from 0.3 to 7.2 percent, although algal lipids are rich in PUFA such as C20:5n3 (eicosapentaenoic acid, EPA) and C22:6n3 (docosahexaenoic acid, DHA). The protein contribution is ranged from 10 to 30 g/100 g dry weight, which may vary greatly among SW species, environmental conditions (especially under nitrogen-enriched condition) and season. The high biological value of algal proteins makes algae suitable for inclusion both in animal feeds (especially marine species) and in human diets. The high carbohydrate content (30 to 60%) is a very marked characteristic in most SW, comprising mainly soluble carbohydrates, like sugars, and pectins, alginic acid, agar and carrageenan as well. Besides their potential nutritional value, from a technological point of view, SW can also be used as additives in the feed industry, for instance, as excellent feed agglutinants (improving texture and water stability of pellets), or as attractants (increasing feed intake).
The effects of seaweeds on fish
Several studies have proved that addition of small amount of SW in aquafeeds resulted in considerable positive effect on growth performance and feed utilisation efficiency, carcass quality, physiological activity, intestinal microbiota, disease resistance, and stress response (Valente et al., 2006). Nonetheless, it has been also noted in other publications that high SW inclusion reduces fish growth and feed efficiency. From the literature available it can be deducted that the response of animals to SW seems to be dose-dependent and species-specific. Moreover, certain substances with antinutritive activity may be present in SW, like lectins, tannins, phytic acid, and protease and amylase inhibitors (Oliveira et al., 2009). Such antinutritional factors might interfere with bioavailability and/or digestibility of nutrients Special emphasis should be focused on protease inhibitors. Binding of protease inhibitors to proteolytic enzymes causes the pancreas to secrete larger amounts of digestive enzymes to overcome the negative effects of inhibitors on the digestion of dietary protein. This fact can lead to decreased weight gain, and pancreatic hypertrophy in some fish species. For this reason, studies aimed to include SW in aquafeeds must also bring up their possible effects on fish digestive physiology. To date, there is scarce literature analysing if SW inclusion causes negative consequences on digestive physiology of fish.
Evaluating the effect of seaweeds on digestive proteases
In a recent study, we evaluated the effect of inclusion of two SW as dietary ingredients on intestinal proteolytic activity of juvenile sea bream. Gracilaria cornea (GR) and Ulva rigida (UL) were chosen in the present study owing to its fast growth, low-cost production and successful integrated culture in fish-farm effluents. Biomass was obtained from the Marine Biotechnology Centre (ULPGC, Spain). SW were cultivated in 750 L semicircular fibreglass tanks filled with seawater plus the fishpond effluents of a pilot aquaculture system (11 m3 with an optimal density of Sparus aurata of 20 kg m-3, and a water renovation rate of 6–8 vol day-1). Red and green SW were washed with sea water,sun-dried for 48 hours, ground and sieved through 0.1 mm sieve before being used as a dietary ingredient.
Dry algal biomass was incorporated into six experimental diets (40% crude protein and 12% crude lipid) at increasing levels (5, 15 and 25%). A feed without SW served as a control diet. Feeds were made at the University of Almeria-CEIA3 facilities (Service of Experimental Diets; http://www.ual.es/ stecnicos_spe). Every experimental feed was randomly assigned to triplicate group of fifteen sea bream juveniles (15.4 g initial body weight). Fish were fed by hand twice per day (9:00 and 17:00) at a rate of 3 percent of their body weight for 70 days. At the end of the trial, fish were killed according to the requirements of the Directive 2010/63/ UE, and digestive tract was removed, and then processes to obtain enzymatic extracts. Intestinal proteases were analysed by two different approaches: a) quantifying the level of intestinal proteolytic activity, and b) visualizing the profile of intestinal proteases in zymograms (Alarcón et al., 1998). In addition, the presence of protease inhibitorsin SW was tested according to Alarcón et al. (1999).
Checking the presence of protease inhibitors in SW
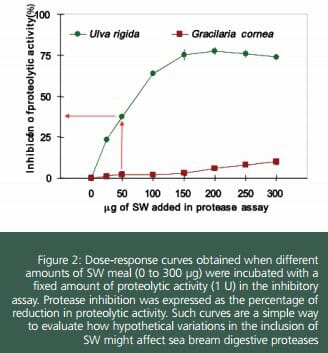
Results revealed the presence of protease inhibitors in SW. Dose-response curves showed that UL contained substances able to reduce digestive proteolytic activity in sea bream (up to 77%), whereas a negligible inhibition by GR was found (4%). Obvious differences in the kinetic of inhibition of protease activity were found for UL. Equation defining such curve may be used to predict the expected percentage of reduction in protease activity, once protease activity in the digestive tract and the amount of feed ingested are known. For instance, in the case of 40 g sea bream, total protease activity released after ameal is around 1,300 units. Those fish that consumed 0.5 g of a feed containing 15 percent of UL, showed a ratio mg UL per unit of activity of 50, which determined a reduction nearly 40 percent in the activity of digestive proteases. Fortunately, fish have mechanisms to compensate the effect of dietary antinutrients.
Zymograms obtained after electrophoretic. separation of proteins is a useful tool to know in detail the type of inhibition caused by protease inhibitors. From the zymogram, it is clear that Ulva produces a generalised inhibition in alkaline proteases of sea bream. On the contrary Gracilaria did not affect any of the active bands.
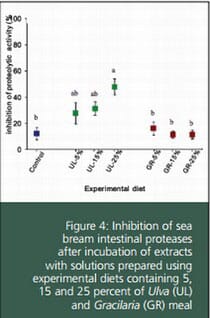
The same results were observed after incubation of digestive proteases with extracts of the experimental diets. The mean inhibition ranged from 11 to 48 percent. In general, UL-supplemented feeds showed inhibition values higher than the GR-supplemented diets, which did not exceed 16 percent. For UL diets, it was found that percentage of inhibition was positively correlated with the SW inclusion level, which agrees with the above mentioned dose-response curve. Inhibition produced by GR feeds cannot be associated to the use of this SW.
Effect of seaweed on digestive proteases of sea bream
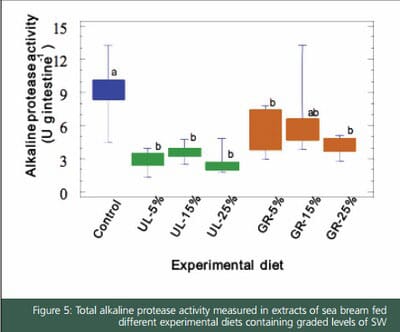
Digestive enzymes were affected by diets, as fish had different enzyme activity level of alkaline proteases after 70 days of feeding experimental diets. In general, a decrease in alkaline protease activity was evidenced when feeds included UL or GR. In particular, the proteolytic activities of fish fed Ulva supplemented-feeds were significantly lower than those of fish fed on control diet. The presence of protease inhibitors in SW may be the reason of the progressive decrease in the proteolytic activity in fish fed diet with increasing levels of Ulva meal. Supporting this hypothesis, it has been confirmed that aqueous extracts of Ulva meal inhibit alkaline proteases of S. aurata. Moreover, the drop in the level of alkaline protease activity was not accompanied by a decrease of fish growth and feed utilization, since all fish grew equally (unpublished data). Santigosa et al. (2008) reported a similar finding when trout were fed on diets including plant proteins.
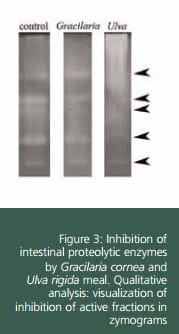
On the other hand, the analysis of zymograms revealed that the pattern of intestinal proteases was not modified by inclusion of SW. All sea bream specimens showed the same number and distribution of active fractions as in control group (after electrophoretical separation, the pattern of intestinal proteases in this species is characterized by five groups of active bands). These results confirmed that the type of alkaline proteases secreted into the intestinal lumen was not modified by any of experimental diets. The existence of a compensation mechanism against dietary protease inhibitors in juvenile sea bream has been previously proved by Santigosa et al. (2010), who found similar results when fish were fed diets with soybean trypsin inhibitor.
According to the results, it is clear that the amount of the pancreatic proteases secreted into the intestinal lumen in juvenile S. aurata is affected by the use of SW, particularly Ulva. Nevertheless, it is also evident that these ingredients did not cause qualitative changes in the composition of alkaline proteases, given that all fish showed the same pattern of proteolytic enzymes in their intestines, and that growth performance of fish was not affected, as deduced from the in vivo feeding trial.
Conclusion
In vitro protease inhibition assays are a useful tool to assess the presence of antinutrients in SW with potential use in aquafeeds. Based on the results of this study, SW, especially Ulva rigida, have antinutritive factors able to inhibit digestive proteases of S. aurata. Feeding juvenile S. aurata on seaweed-based diets decreased the amount of proteolytic activity secreted into the intestine.
However, the inclusion of SW does not alter the pattern of proteolytic enzymes in sea bream, which reveals a compensating mechanism in this species.Research is being currently conducted to assess the effect of SW on other digestive enzymes, intestinal microbiota, blood and tissue metabolites, and intestine and liver histology after 70 days of feeding SW-based diets. Further research is needed in order to known the impact of SW in a long-term feeding assay.
October 2013