To date, there is very few standardized methods among the controlled reproduction protocols in pikeperch, Sander lucioperca, (e.g. Zarski et al., 2012a). This includes the in vitro fertilization protocol, which was investigated in a low extent (Krištan et al., 2012). Effectiveness of in vitro fertilization is dependent, among others, on the time of contact of gametes (egg and sperm) with each other (e.g. Zarski et al., 2012b). That is why, the knowledge on the period of time during which the gametes retain the fertilization capability is of high importance.
The aim of this study was to evaluate the period of pikeperch eggs ability to fertilization following exposure to hatchery water.
Material and Methods
The eggs obtained from three hormonally stimulated (hCG, 500 IU kg-1) wild pikeperch females were used in this study.
After the ovulation occurred, eggs were stripped into a dry plastic container and covered immediately. Experiment was performed on the eggs of three females separately and every time with the use of fresh pooled (from three males) sperm.
For the fertilization trial, about 200 eggs were placed on the Petri dish (50 mm diameter) with 10 mL of hatchery water (14°C) and next the sperm sample (50 μL) was added at 0 (control group), 15, 30, 60, 90, 120, 150 and 180 s post eggs activation. The eggs with the sperm was stirred for about 1 min post activation and then left for about 5 min before the samples were placed in an incubation tank. Eggs were incubated on Petri dishes in a recirculating aquaculture system at 14°C. Embryos survival was determined after 24 h of incubation, since after this time it was easily possible to distinguish the live and dead embryos. To determine embryonic survival, each Petri dish was photographed (two fields of view each) under a stereoscopic microscope (Leica, MZ 16.5, Switzerland) what allowed further analysis.
The results of embryonic survival (after Arc-Sine transformation) at a respective time was analyzed with the on-way analysis of variance (ANOVA) at a significance level of 5% (α=0.05). Additionally, Pearson’s correlation was applied for the same set of data.
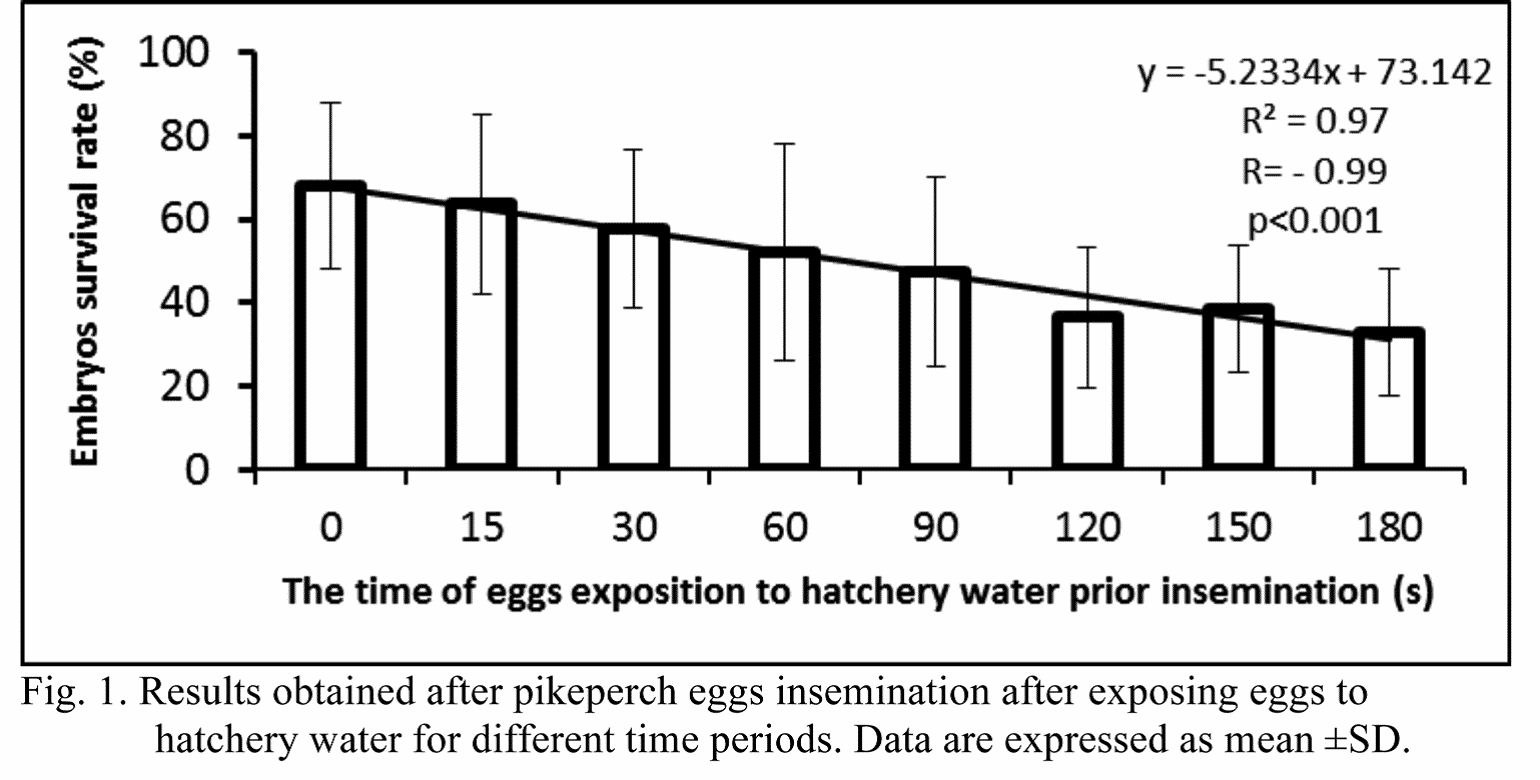
Results
In the control group (0 s post activation) the highest survival rate of embryos was noted (67.9%). The lowest embryonic survival rate was found in the group where sperm was added after 180 s post eggs activation (32.9%). Despite the significant negative correlation (r= –0.99; p<0,001) of the time after activation and the embryonic survival rate, no significant differences (P>0.05) were found between the embryonic survival in the control group and the eggs inseminated at 180 s post its activation (Fig. 1).
Discussion
The duration of eggs ability to fertilization varies among the species. For example, eggs of the Eurasian perch, Perca fluviatilis L., were able to be fertilized very effectively (without significant decrement in fertilization rate) for 150 s (Zarski et al., 2012b). On the other hand, the eggs of crucian carp, Carassiu carassius, significantly lost its fertilization capability after 60 or 120 s post activation in a reverse osmosis water and Woynarovich solution (4 g NaCl and 3 g urea per liter), respectively (Zarski et al., unpublished).
The results obtained in this study did not reveal any significant differences (P>0.05) within the 180 s. However, it resulted from the high variability in the eggs quality which masked the possible differences. Such variable eggs quality was very often observed in the case of the wild pikeperch (Zarski et al., 2012a).
The correlation (r= –0.99; p<0,001) clearly showed, that within the considered period of time there is highly decreasing tendency in the survival rate of embryos when eggs are inseminated after a certain period of time. This is, in contrast to the Eurasian perch, where insemination was recommended to perform after at least 15 s post eggs activation, what significantly improved the fertilization rate (Zarski et al., 2012b).
However, such difference results probably from the form of the eggs spawned by the two species. In Eurasian perch eggs are situated within the cylindro-conical “ribbon” whereas eggs of pikeperch are ovulated as a batch of single eggs. Therefore, it may be concluded, that eggs of the pikeperch should be inseminated immediately after activation and every delay in sperm addition may negatively affect the fertilization result.
October 2013



