Both sexes were individually stocked into hapas (enclosures) and fed a basal diet supplemented with graded levels 0.0, 0.5, 1.0 and 2.0 g Therigon® kg-1 diet (T1-T4; T1 as a control treatment, respectively); 1.0, 2.0, and 3.0 g Nuvisol Hatch P® kg-1 diet; (T5-T7); 20, 40 and 60 mg gibberllic acid kg-1 diet (T8-T10) for female only and 700, 900 and 1100 mg L-carnitine kg-1 diet for males only (T12-T14), respectively for 19 day. Results revealed that fish ovaries in all treatments (T2-T10) contained more advanced oocytes at final maturation stage with clear histological alterations of fish fed the highest levels of these feed additives, especially with gibberllic acid treatment. Fish treated with 60 mg gibberllic acid kg-1 diet (T10) showed the worst histological effects on fish ovary among all treatments. Addition of 700 and 900 mg L-carnitine kg-1 diet caused maturation of testes better than the control treatment (T11). Meanwhile, fish fed 1100 mg L-carnitine kg-1 diet (T14) showed degeneration and severe hemolysis in seminiferous tubules. Low bacterial loads were recorded (in gills, liver, intestine and ovary / testes) at the high level of Nuvisol Hatch P®. Data proved that the high bacterial load was detected in fish treated with L-carnitine in both tested media and in all tested organs. Meanwhile, low bacterial load was observed in the liver, ovary and testes in all media comparing with the other organs (gills and intestine).
Introduction
Nile tilapia (Oreochromis niloticus) are considered as the most common and popular fish in Egypt. Egypt is a country where, arguably, the farming of tilapia has its roots (Stickney, 2006). Latest fish production statistics in Egypt revealed that tilapia constitutes 52.86 per cent of the total fish production in 2010 (GAFRD, 2010) and occupy the 10th order concerning the world production from aquaculture (Van Hauwaert et al., 2000). Hence, Egypt produces 20 per cent of the world tilapia catch and 12 per cent of the world farmed tilapia (EL-Sayed, 2006). The culture of O. niloticus in Egypt is still growing in a remarkable way with apparent intend towards intensification that pressurizing the need of enormous number of seeds to satisfy the needs for aquaculture, so rigorous management of large numbers of brood stock are necessary for mass production of eggs and fry due to relatively small number of eggs produced per spawning.
Many limitations associated with tilapia fry production under the prevailing Egyptian conditions were described by El-Gamal (2002). Furthermore, it is widely accepted that effective seed production demands a thorough understanding of the special husbandry and particular nutritional requirements of brood stock fish which significantly affect fecundity, survival, egg size, fertilization percentage and larval hatchability quality (Bromage, 1998). Therefore, the present research aimed to evaluate the powerful of three commercial synthetic feed additives (Therigon®, Nuvisol Hatch P® and gibberllic acid) for improving the reproductive performance of females Nile tilapia brood stock, and fourth one (L - carnitine) for males from the view point of the gonadal structure characteristics and microbiological examination.
Materials and methods
Experimental management:
A field study was conducted in a private earthen pond fish farm located at Alabhar belonging to Alhamol, Kafr Alshiekh governorate. Fourteen hapas (each 3 m width × 6 m length × 0.5 m water depth and 1 m total depth) were constructed in a two feddans earthen pond. The first ten hapas were stocked with O. niloticus females' brood stock of one year with an average body weight of 150 g from the same farm. The other four hapas were stocked with males' brood stock of (the same age as the females, but of an average body weight of 200 g) from the same farm also. Each hapa was stocked with twenty fish. They were incorporated in a feeding trial to test the effects of some commercial dietary additives on fish propagation. The experimental feeding period (19 days) began on 20 June 8 July, where the feed was offered to fish twice daily, at a daily feeding rate of 3 per cent of the fish biomass in each hapa. The feed additives were purchased from the local market and added directly to a mash diet, which was purchased also from the local market (contained 90.31 per cent dry matter, 80.88 per cent organic matter, 23.81 per cent crude protein, 5.47 per cent ether extract, 51.60 per cent carbohydrate and 9.43 per cent ash) and moistened to be pelleted via a hand mincer.
Dietary treatments:
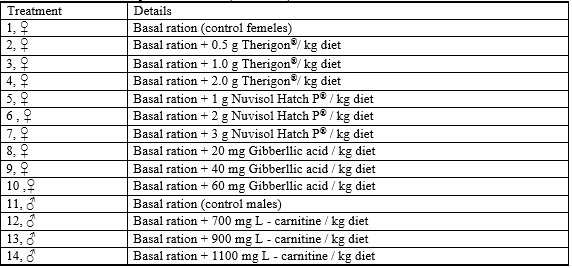
Fish were fed on a basal ration (BR) with or without (control) the tested feed additives as illustrated in Table 1 which were:
- Therigon® powder for veterinary use, manufactured by Adwia Co., S. A. E. 10th of Ramadan city, Egypt. Each 1 g contains Alpha – Amino – p – hydroxyhydrocynnamic acid, 1000 g package as GnRH stimulant (Batch No. 0601116).
- Nuvisol Hatch P®, imported by Khirat Alnile Co., 27 Alferdos Buildings, Flat 43, Nasr city, Egypt from Newtrix Co., Belgium, in 500 g package. Each 1 Kg contains the following vitamins (in mg): B1 4000, B2 5000, B3 4000, B6 2000, B9 1000, B12 20, PP 10000, Biotin 50, and L – carnitine 30000.
- Gibberllic acid (C19 H22 O6), type analysis, Art. 3930, M. W. 346.38, M. P. 225 °C, GA3 content 90 per cent, 1 g package, Batch No. 43124, imported from Lobal Chemie, Pvt. LTD, 2042 Bombay, India.
- L – carnitine powder from Mebaco, Egypt.
Criteria measured:
After the 19 days feeding trial of the separate sexes of brood stock, fish samples were caught from each hapa for the histological examination of the gonads, and for microbiological tests for gills, livers, intestine and gonads.
Histological examination:
At the end of the experiment, fish were sacrificed and the gonads were sampled. Samples were fixed in 10 per cent neutralized formalin solution followed by washing with tap water, then dehydrated by different grades of alcohol (70, 85, 96 and 99 per cent). Samples were cleared by xylene and embedded in paraffin wax. The wax blocks were sectioned into six micron. The sections were stained by hematoxyline (H) and eosin (E) and then subjected to a histological examination according to Roberts (2001).
Table1: Details of the experimental diets (treatments)
Microbiological examination:
Bacteria were identified by general and specific media and biochemical testes, the following were the cultivation media used for different purposes.
1- Nutrient agar (NA, as a general medium). 2- Salmonella / Shigella agar (SSA). 3- Brucella agar (BR). 4- Baird-parker agar (BP). 5- Trypticas soy agar (TSA). Three fish from each hapa were used for bacterial counts in fish organs (gills, liver, intestine and ovary/ testes). The fish were killed by physical destruction of the brain and the number of incidental organisms was reduced by washing the fish skin with 70 per cent ethanol before taking the gills and opening the ventral surface with sterile scissors to expose the body cavity. From each fish, 1 g of each tested organ was removed and suspended in 10 ml of sterile saline (0.85 per cent (w/v) NaCl). The suspension was serially diluted to 103, and 1 ml of the solution was spread on each medium plates. The inoculated plates were cultured as described above and the number of colonies was counted. Appropriate serial dilutions of fish and water samples were then plated onto plate count agar.
Aerobic plate count (APC):
For total heterotrophic aerobic bacterial counts of gills, liver, intestine and ovary/ testes of tilapia, all the inoculated plates were incubated at 30°C for 48 h and colony forming units (CFU) were counted. Readings obtained within the range 30 to 300 colonies on a plate were used to calculate bacterial population numbers, recorded as CFU per g of sample. The bacterial colonies were divided into different types according to the colony characteristics shape, size, color and opacity, and the number of colonies of each recognizable type was counted. Three to five representatives of each colony type were then streaked on additional NA plates repeatedly until pure cultures were obtained.
Identification of bacteria:
All the purified isolates were observed for cell shape, spores, and gram stain reaction. The isolates were then subjected to biochemical identification following the criteria described in the Bergey’s Manual of Systematic Bacteriology (1st ed.) (Holt et al., 1994) for identification to the genus or the species level.
Statistical analysis:
Data of the bacteriology were statistically analyzed using coefficient of variance, cross tab, and Chi-Square test, which were described by Bailey (1995).
Results and discussion
Histological examinations:
A) Ovaries
The histological studies of ovarian tissues of O. niloticus (Figs. 1 & 2) fed the basal ration (BR) with or without the tested feed additives (T2-T10, and T1, respectively) showed three developmental stages of oocytes during oogenesis. These stages include cortical alveoli formation (yolk vesicle stage), vitellogenic (yolk) stage and ripe or mature stage, while few oocytes appeared at early stages.
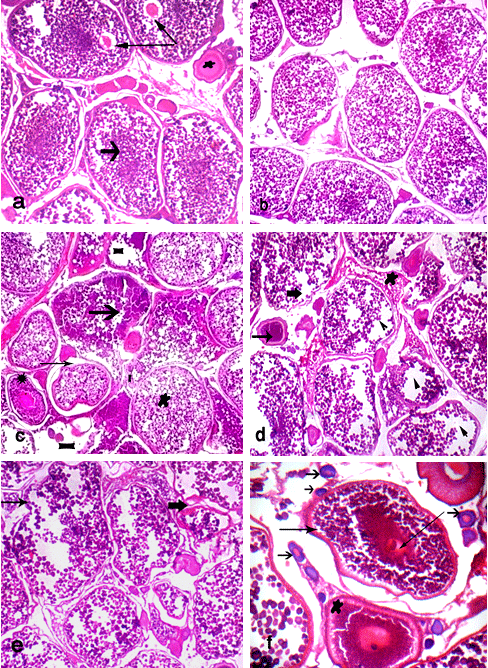
Fish fed BR (control) showed normal structure of ovarian tissues; it showed ripe stage of oocyte with migratory nucleus and start of yolk liquefaction of cytoplasm of oocyte and large connected vacuoles of some oocytes (Fig. 1a). Ovary of fish fed the BR plus 0.5g Therigon/ kg diet (T2) showed ripe stage of oocyte (Fig. 1b). While the ovaries of fish fed the BR plus 1.0g Therigon/ kg diet (T3) showed oocyte in yolk vesicle stage (in normal structure) beside many oocytes in ripe stage. In addition, clear histological lesions were noticed in ovary as coagulative necrosis in yolk granules, separation of follicular layers, degeneration in oocyte (atresia) and focal area of necrosis in ovarian tissues, where the ovarian tissues were found to consist of interstitial cells, adipose cells, yolk granules and blood capillaries (Fig. 1c). Degenerative changes (atretic follicles) were observed in the ripe oocytes in ovary of fish fed the BR plus 2.0g Therigon/ kg diet (T4) with depletion of the yolk granules (Fig. 1d). So, the ovaries in fish fed with diets supplemented with 0.5g Therigon/ kg diet were protected to be atretic comparing with those fed high levels of Therigon (1.0 & 2.0g / kg diet).
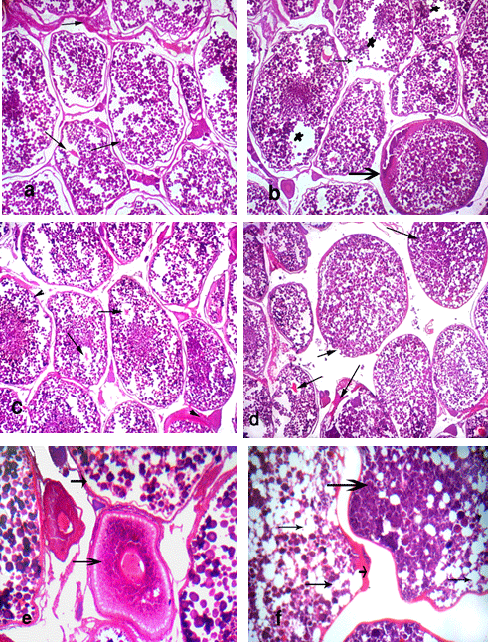
Fish fed the BR plus 1.0g Nuvisol Hatch P® / kg diet (T5) showed that the ovary becomes more developed and reached to the final maturation (ripe oocytes) stage with few atretic follicles (Fig. 1e). The level of 2.0g Nuvisol Hatch P® / kg diet (T6) showed different development stages of oocytes (chromatin nucleolar stage, perinucleolar stage, cortical alveoli formation stage, vitellogenic stage, ripe stage) with irregular walls of oocytes (Fig. 1f). Whereas the high level of Nuvisol Hatch P® (3.0g / kg diet, T7) showed ripe oocytes stage with migratory nucleus which has an irregular outline, besides hemolysis in the ovarian tissues (Fig. 2a). Concerning the ovaries of fish fed the gibberllic acid at the 3 levels (T8 - T10, Fig. 2, b - f), T8 and T9 showed the ovary in ripe stage with slight histological changes of oocytes and liquefaction of cytoplasm of oocyte with nucleus loses its circularity and degeneration in oocyte wall (Fig. 2, b & c). Yet, fish fed the BR plus 60 mg /kg diet gibberllic acid (T10) shows the ovary in ripe stage and vitellogenic oocyte (Fig. 2, d & e) with histological alterations including erosion in walls of some oocytes, coagulative necrosis, liquefaction of the yolk sphere with large vacuoles of ripe oocyte of O. niloticus, and irregular wall of oocytes (Fig. 2, d & f).
Ovaries of fish fed the two high levels of tested feed additives showed histological alterations. Furthermore, fish treated with dietary supplementation of gibberllic acid showed the worst effects on fish ovary among all treatments. In contrast, the low level of these feed additives showed no clear histological changes of ovary, especially 0.5g Therigon/ kg diet (T2) which reflected the high level of FSH (91 mIU /ml) among all treatments (Abdelhamid et al., 2010; a complementary study of the present work). According to Nagahama et al. (1995 a & b), vitellogenesis and oocyte maturation are regulated primarily by FSH and LH, respectively.
The histological changes in the ovaries of O. niloticus observed in some treatments may be attributed to many causes, such as a mechanism to regulate fecundity may be due to the tested feed additives or to environmental stress (as water pollution), where the different pollutants such as agriculture wastes and different types of bacteria have histological effects on the reproductive tissues of fish gonads (Lye et al., 1998). These effects may disturb the development of germ cells and may reduce the ability of fish to reproduce.
Furthermore, Abdelhamid et al. (2010) confirmed that dietary inclusion of 1 g Nuvisol Hatch P® / kg diet (T5) and 1 g Therigon®(as GnRH stimulant) / kg diet (T3) realized good female's reproduction performance. Gonadotropin releasing hormone (GnRH) is a hypothalamic neuronal secretory decapeptide that plays a pivotal role in reproduction. GnRH analogue stimulates gonadotropin secretion (Van Der Kraak et al., 1983) steroidogenesis (Van Der Kraak et al., 1984) and ovulation (Haraldsson and Sveinsson, 1993) in salmon and in many other species. In fish, gibberllic acid (GA3)at low levels improved Nile tilapia growth and gonado-somatic index (Abdelhamid et al., 1998), since it is a nitrogenous compound with estrogenic effect; where it increased the percent of egg production, hatchability and ovary and oviduct relative weight significantly (El-Sebai et al., 2003). So using natural GA3 instead of the synthetic estrogen is safer and environmental friendly, therefore should be considered.
B) Testis
Testis of Nile tilapia brood stock fed the basal ration only (T11, as a control) showed normal structure of seminiferous tubules with spermatocytes, spermatids and few sperms (Fig. 3, a & 3). However, dietary supplementation of L-carnitine caused histological maturation of testis of treated fish especially at level of 700 mg (T12), which showed normal structure of seminiferous tubules filled with spermatozoa, spermatocytes and spermatids (Fig. 3, c & d). In case of the fish fed 900 mg L-carnitine / kg diet, testes showed normal structure of seminiferous tubules filled with spermatids and spermatozoa at ripe stage (Fig. 3e).
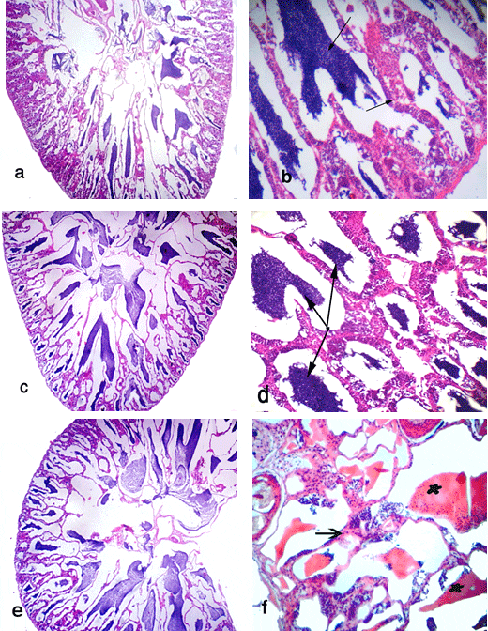
Meanwhile, fish fed the basal ration plus 1100 mg L-carnitine / kg diet (T14) showed extensive degeneration of the seminiferous tubules, necrotic changes in the cellular elements of the seminiferous tubules and focal areas of necrosis with severe hemolysis of testes' tissue. In addition, testes of this group appeared free from spermatocytes or spermatids among all treatments (Fig. 3 f). These drastic histological alterations in testes may be related with increasing level of L-carnitine in fish diet. In addition, all of these histological alterations in testis are due to the commercial feed additives tested in the present study.
Several stages of spermatogenesis (spermatocytes, spermatid and spermatozoa) in the present study are similar to those reported by Msiska (2002). In addition, secondary spermatocytes were illustrated by darkly chromatin staining as in other teleost fish. Meanwhile, spermatozoa were concentrated in the lumen (Tyler and Sumpter, 1996). Furthermore, Abdelhamid et al. (2010) confirmed that dietary supplementation with 700 (T12) and 900 (T13) mg L-carnitine / kg diet gave better results of males' reproductive performance. L–carnitine is a naturally containing amino acid derivative (dipeptide amino acid), synthesized from methionine and lysine. L–carmitin, a betaine derivative of β – hydroxybutyrate, could be biosynthesized in plant and animal cells via lysine, methionine, and some vitamins like B6, C, nicotinic acid and folate (Zeyner and Hameyer, 1999).
Since L–carnitine provides an energetic substrate for the spermatozoa in the epidydimis, it contributes directly in sperm motility and may be involved in the successful maturation of the sperm (Chatzifotis et al., 1995). L–carnitine improved semen quality and histological characteristics of the testes (El-Damrawy, 2007). Generally, a low level of L–carnitine enrichment provides several protective effects in fish reared under intensive pond-culture conditions (Harpaz, 2005). Also, Jayaprakas et al. (1996) reported that supplementation with 900 mg L-carnitine / kg diet had a positive effect on the reproductive performance of male Mossambique tilapia, significantly increasing both testes weight and sperm cell concentration per ml compared with the control.
Count and identification of pathogenic bacteria in gonads and other fish organs:
The results of the total count of bacterial isolates on different media from gonads of O. niloticuss among different treatments are shown in Tables 2 and 3. Data exhibited absence of bacterial loads of ovaries in fish fed the diets supplemented with the three different levels of Nuvisol Hatch P®, among different tested media, So, Nuvisol Hatch P® may have a protective effect against bacterial infection; in contrast, the high bacterial load appeared in testes on BR and BP media with the two high levels of L– carnitine (900 & 1100 mg / kg diet; T13 &T14). Only T13 showed that SSA appeared total count 60 x 103 CFU/g, this media has given an appreciable count of Salmonella sp., Shigella sp., Proteus sp. and Escherichia coli. All these genera are belonging to the family Enterobacteriaceae which are capable of inducing gut wrenching gastroenteritis as reported by Anon. (2005). The biochemical tests (Table 4) showed that the isolated bacteria from testes were Bacillus sp., Salmonella sp and Brucella sp. which were found in T13 more than T14. So, the histological alterations of testes in T14 attributed to the treatment with high level of L– carnitine (1100 mg/ kg diet) not to the contaminated bacteria.
Table 2: Bacterial count (CFU/g) of ovary /testes of O. niloticus isolated on different media from different experimental treatments.
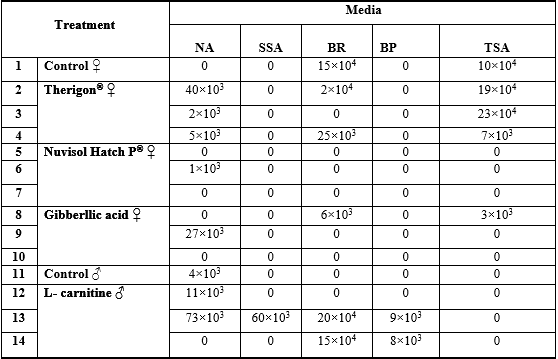
Table 3: Comparison of bacterial count obtained on different media in fish organs from different experimental treatments (means, ×103).
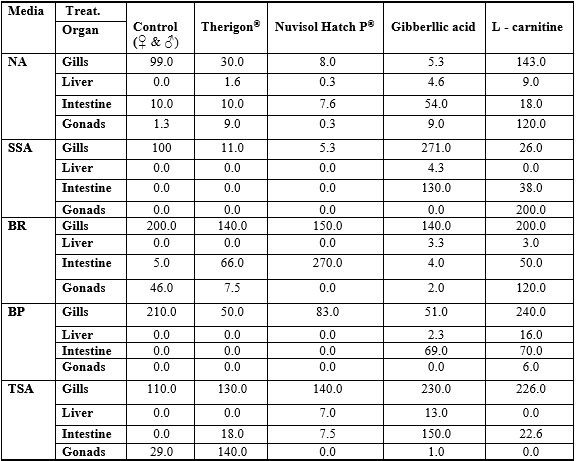
Table 4: Morphological and biochemical characteristics of the isolated bacteria from fish samples which obtained on different media
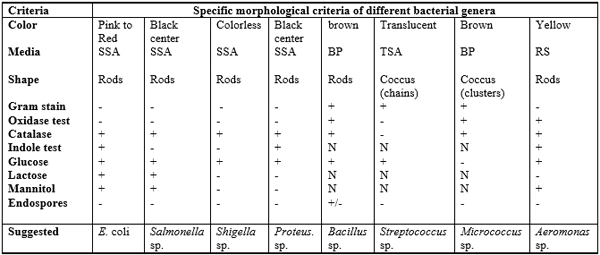
The typical colonial morphology on SSA medium is as follows: both Salmonella and Proteus sp. produce colorless colonies with black center, Shigella colony is colorless (Bopp et al., 1999), while E. coli colony is pink to red. Baird- parker agar (BP) culture medium was specially used for isolation and identification of Staphylococcus sp., Micrococcus (coccoid bacteria) and Bacillus (Bacillus is belonged to family Bacillaceae). Staphylococcus produces black colony on BP medium while Micrococcus and Bacillus species produce brown colony on the same medium. Brucella agar (BR) medium was used for the isolation and cultivation of Brucella that is coccobacilli and belonging to the family of Brucellaceae. Trypticas soy agar (TSA) medium was specially used for isolation of Streptococcus. Some biochemical testes were carried out for identification of these isolated colonies of bacteria as shown in Table 4.
Data in Table 5 represent the suspected bacteria found in various treatments and different organs of fish as well as their cross (Table 6, Chi square). The results showed that the high bacterial load was observed in both gills and intestine on all media comparing with the other organs (liver and ovary /testes), this agrees with Austin (1982) who reported that most of the microorganisms in fish tissue are thought to result from surface, gills, or intestinal contamination, where microorganisms are adsorbed on the surfaces of the fish and found in their intestinal contents. So the initial microbial flora on the caught fish is dependent upon the contamination of the water, bottom sediment from the area of catch and the food entering the digestive tract which may contain microorganisms. In addition, the total count of bacteria obtained on SSA and BP media was lower in all tested organs than the other media (NA, BR and TSA). The statistical comparison of bacterial load of the tested organs among different treatments showed some observation (Table 3).
Table 5: Isolated bacteria from fish organs from different treatments, according to specific media
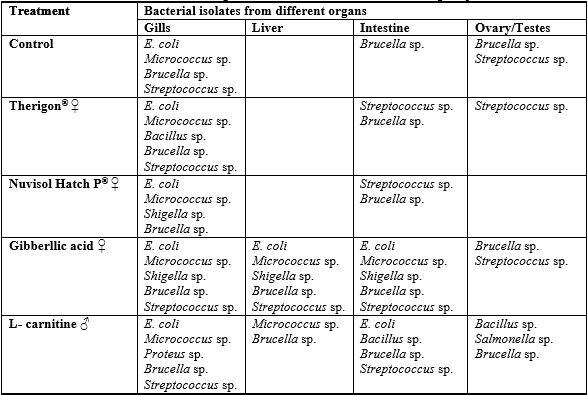
Table 6: Cross table of the examined organs as affected by the dietary treatments
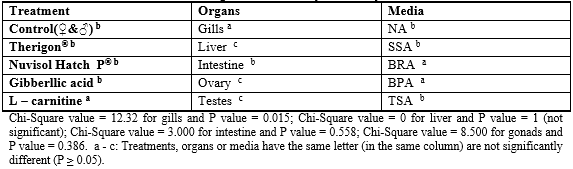
No significant differences were found among tested organs (except gills) and treatments. Generally, tabulated data proved that high bacterial load was detected in fish treated with both of gibberllic acid and L-carnitine on the tested media and in fish organs. So, there was a close correlation between histological alterations of fish gonads which fed on gibberllic acid and L-carnitine diets and the high bacterial load, especially with presence of Salmonella in the testis (Table 5).
The presence of Enterobacteriaceae in some fish organs in this study may be attributed to the rearing water of fish (sewage), Austin and Austin (1987) reported that fish harvested from water polluted with human and animal wastes can contain Salmonella, Shigella, Proteus and Escherichia coli which are usually found among the most prevalent bacteria on the most rearing water of fish. E. coli is still the most widely used indicator for faecal pollution (Snieszko and Axelrod, 1976). Also, Streptococcus sp. are opportunistic pathogens in aquaculture and their pathogenicity depends on environmental stresses as low dissolved oxygen levels, high nitrite concentration (Bunch and Bejeramo, 1997), water hardness, and crowding with water temperatures exceed 20ºC (Ohnishi and Jo, 1983). All vital organs of fish stock infected with streptococci became heavily infected and mortality becomes massive (50 - 60 per cent; Hubbert, 1989).
Streptococcus has been associated with serious economic losses among cultured freshwater and saltwater fish in many parts of the world, especially among tilapia fish. El-Gawady (2002) counted up to 1.9 x 106 – 2.4 x 106 CFU/ml total aerobic bacterial in water of fish farm in winter and summer, respectively. However, the gut and skin of O. niloticus microbial count reached 4.3 x 108 CFU/g. Ampofo and Clerk (2003a) identified 25 species of bacteria as associated with the fish culture systems. Ampofo and Clerk (2003b) also added that maturing causes organic enrichment; it may also hasten the deterioration of the water quality making the aquatic environment favorable for the growth and multiplication of human pathogenic bacteria. Moreover, Abdelhamid et al. (2006 and 2007) registered the presence of pathogenic bacteria (1.3 – 2.0 x 105) in samples of water, feed, sediments and fish, mainly in summer season. There was no difference between fish of natural resources and those of aquaculture concerning bacterial contamination. Also, Abdelhamid et al. (2008) found pathogenic bacteria (X 106 CFU) in samples of water, feed, intestinal, and liver of Nile tilapia up to 113.0, 38.7, 23.33, and 25.00, respectively. However, more recently, Abdelhamid et al. (2012) did not find critical counts of bacteria, whether in water, diet or fish tissues. Also, there were no relations among the counts in water, diets, muscles and/or liver of the tested tilapia fish. Yet, Abd El-Shahid et al. (2009) found that all fish (including Nile tilapia) samples (100) collected from Alexandria fish markets were contaminated with Enterobacteriaceae (5.14 × 103) and coliform (1.02 × 103). Moreover, Shaltout et al. (2009) counted the total microbial count including fungi, yeasts and highest total bacterial counts (TBCs) as indicators for faecal pollution in Kafr El-Zayat industrial area.
Generally, it can be noted that some histopathological signs in gonads and microbiological findings in some organs of the experimental fish were observed. It may be related to fish rearing water quality, as agriculture drained water, which was polluted with different types of pesticides, fertilizers and heavy metals residues not only in this fish farm, but also in all fish farms and fish hatcheries in Egypt according to the Egyptian law No. 124/1983. Currently, this law prohibits aquaculture projects from drawing surface water, leaving more than 90 per cent of the country's fish farms and fish hatcheries to operate on polluted agricultural drainage water.
In conclusion, the obtained results revealed that low levels of all feed additives (Therigon®, Nuvisol Hatch P® and gibberllic acid for females and L - carnitine for males) caused maturation of ovaries and testes better than the high levels. In addition, the high bacterial load was detected in fish treated with the highest levels of gibberllic acid and L-carnitine in all tested fish organs. So, it can be recommended that the Egyptian government must be mending this dishonorable law (No. 124/1983) for sustainability development in fish production sector and human health considerations.
January 2014