Global mean atmospheric carbon dioxide (CO2) concentrations have increased by nearly 40 per cent from 280 parts per million (ppm) during preindustrial times to approximately 390 ppm by the year 2010 (Doney, 2010). The equivalent of nearly 30 per cent of total human-derived CO2 emissions have entered the world’s oceans, altering ocean chemistry and leading to more acidic seawater with lower carbonate ion [CO2− 3 ] concentrations (Sabine et al., 2004). Studies have demonstrated that this “ocean acidification” affects a spectrum of organismal traits, including but not limited to growth, reproduction, development, shell strength, skeletal composition, and survival (e.g., Kurihara et al., 2007; Gaylord et al., 2011; LaVigne et al., 2013), across a diversity of taxa (Doney et al., 2009; Kroeker et al., 2010, 2013; Gazeau et al., 2013). There have been fewer studies examining how impacts might be influenced by accompanying shifts in other environmental factors; most have focused on combined effects of altered pH/pCO2 and temperature (reviewed by Byrne, 2011). However, other environmental factors, such as salinity, dissolved oxygen, and patterns of primary production are also predicted to diverge from present values (Behrenfeld et al., 2006; Harley et al., 2006; Riebesell et al., 2007; Diaz and Rosenberg, 2008; Doney, 2010).
The consequences of such multifaceted changes may be particularly dramatic in nearshore environments. This could be especially true in areas where the upwelling process, which brings waters that are naturally pCO2-rich to the surface, already exposes coastal benthic marine invertebrate species to waters low in pH and carbonate concentrations (Feely et al., 2008, 2010; Barton et al., 2012; Gruber et al., 2011, 2012). Moreover, some models and time series data project an enhancement in the intensity of upwelling, as may be the case for the California Current System of the eastern Pacific (Bakun, 1990; Snyder et al., 2003; García-Reyes and Largier, 2010). Altered water temperature and nutrients, coupled with ocean acidification, may influence phytoplankton productivity (Rose et al., 2009), a food source for pelagic planktotrophic larval stages (Phillips and Gaines, 2002).
In marine systems, the planktonic food environment influences larval condition, and is one of a suite of interacting variables that affects larval and juvenile quality (Phillips, 2002; Boidron-Métairon, 1995; Pace and Manahan, 2007; Giménez, 2010; Vargas et al., 2006, 2013). Furthermore, the ability of planktotrophic larvae to ingest a range of food particle sizes and types (e.g., Baldwin and Newell, 1995) could influence larval responses to ocean acidificationinduced shifts in the biochemical and taxonomic composition of phytoplankton. There are mechanisms by which ocean productivity could moderate ocean acidification impacts on planktotrophic larvae. First, ocean acidification may induce changes in phytoplankton growth, inorganic carbon uptake, nitrogen fixation, and elemental ratios (Berge et al., 2010; Reinfelder, 2012), each of which could reduce the nutritional food quality for planktotrophic larvae and other consumers (Riebesell et al., 2007; Rossoll et al., 2012; Reinfelder, 2012). Secondly, ocean acidification could also alter the taxonomic composition of phytoplankton assemblages through a combination of direct and indirect effects (Rose et al., 2009; Caron and Hutchins, 2013). Such shifts may be important because larval performance in oysters and other invertebrates is often sensitive to phytoplankton composition (Thompson et al., 1993; Talmage and Gobler, 2012; Vargas et al., 2013). These shifts would operate in addition to upwelling-induced phytoplankton blooms directly altering seawater chemistry by drawing down total CO2 through enhanced rates of photosynthesis, shifting carbonate and pH levels back towards conditions favorable for marine invertebrate shell and skeleton-building (Hauri et al., 2009).
Surmounting low pH conditions can incur increased energetic costs, and thus changes in food supply might modulate the effects of ocean acidification on marine taxa across life history stages. Nevertheless, few experiments have been performed to determine whether improved nutrition might ameliorate the negative effects of ocean acidification or whether impaired nutrition might exacerbate these effects. Among the studies that have been conducted on bivalves, Melzner et al. (2011) found greater internal shell dissolution of adult mussels when individuals were exposed to high pCO2 and low food density. Examining a different life stage of the same mussel species, Thomsen et al. (2013) found that mussels were able to overcome exposure to high pCO2 during the juvenile phase if there was sufficient food available in the environment.
A remaining unknown is how larval responses to elevated pCO2 might change as a function of food availability. Ocean acidification has been shown to affect larvae and the larvalto- juvenile transition in bivalves (e.g., Talmage and Gobler, 2009; Parker et al., 2010; Barton et al., 2012), and research indicates that energetic stresses imposed during the larval phase (including those from elevated pCO2) can influence latter life stages (e.g., Rodriguez et al., 1990; Videla et al., 1998; Pechenik, 2006; Hettinger et al., 2012, 2013). Less clear is the degree to which plentiful food might counteract any or all of these impacts. To address this, we exposed larval Olympia oysters (O. lurida) to a factorial combination of food and pCO2 regimes, tracked growth over the full pelagic duration, and determined percent metamorphosis.
Results
Seawater chemistry during larval culturing
pH values in the pCO2 treatments differed from one another (ANOVA, pCO2, F1,12 = 22850.5, p < 0.0001; Table 1). pH of jars within headspaces assigned to the same treatment also varied slightly (ANOVA, headspace [pCO2], F4,12 = 6.66, p < 0.005). Specifically, mean pH in the jars from headspace 1 in the 500 μatm pCO2 treatment differed from headspaces 2 and 3 by 0.009 pH unit. pH levels across the three headspaces in the 1000 μatm pCO2 level did not differ. pH values also differed as a function of food level (ANOVA, food, F2,12 = 33.82, p < 0.0001; Table 1). pH units decreased approximately 0.01 unit between each of the subsequent food levels in both pCO2 levels (i.e., the high food level had a pH unit 0.01 lower than the medium food level and the medium food level had a pH unit 0.01 lower than the low food level). This pattern of decreasing pH with increasing food levels (i.e., algal concentrations) was likely from increased respiration in treatments with higher concentrations of algae, given the low-light conditions that characterized our cultures. Total alkalinity did not differ between the pCO2 treatments (ANOVA, pCO2, F1,12 = 0.38, p = 0.547), among food levels (ANOVA, food, F2,12 = 0.41, p = 0.675) (Table 1), or among the headspaces assigned to the same treatments (ANOVA, headspace[pCO2], F4,12 = 0.30, p = 0.871). DIC samples were used simply to overconstrain the carbonate system and were not analyzed statistically.
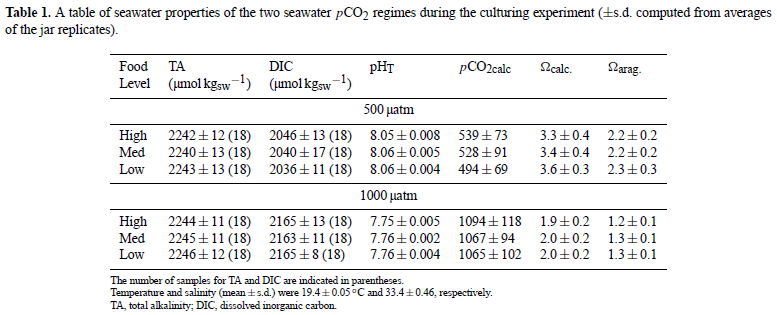
Larval growth
Larval growth on day 5 post-larval release was reduced in low compared to medium food levels (ANOVA, p = 0.039), but did not differ between the two pCO2 concentrations (ANOVA, p = 0.092; Table S1, Fig. 1a). Similar patterns were exhibited on day 9; however, at this time point, growth was reduced in low compared to medium and high food levels (ANOVA, food, p = 0.002; pCO2, p = 0.175; Table S2, Fig. 1b). At day 11, growth was reduced in low food compared to medium and high food levels (ANOVA, p = 0.048) and also in high compared to control pCO2 conditions (ANOVA, p = 0.047; Table S3, Fig. 1c). Indeed, larval growth was 9 per cent, 5 per cent, and 14 per cent higher in control compared to high pCO2 conditions, for the high, medium, and low food treatments, respectively. The effects of food on larval growth were consistent regardless of pCO2 concentration (ANOVA, pCO2 ×food, Day 5, p = 0.778; Day 9, p = 0.962; Day 11, p = 0.720; Table S1–S3). Larval growth did not vary between headspaces assigned to the same treatment (Table S1–S3).
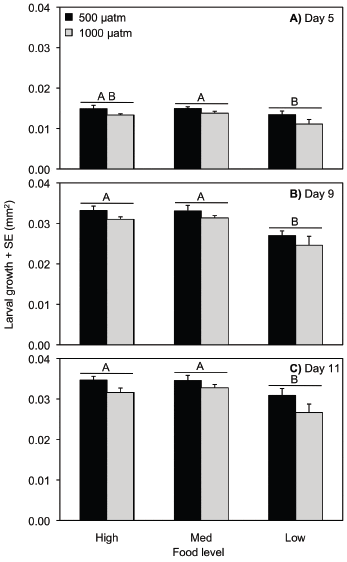
Larval total dry weight
On day 5 post-larval release, total dry weight was reduced in low compared to medium food levels (ANOVA, p = 0.001) and was also reduced in elevated compared to control pCO2 conditions (ANOVA, p = 0.002; Table S4, Fig. 2a). On day 9, low food decreased total dry weight compared to medium and high food levels (ANOVA, p = 0.003), but pCO2 concentration did not have an effect (ANOVA, p = 0.206; Table S5, Fig. 2b). The same patterns were exhibited on day 11 for low food (ANOVA, p < 0.0001) and pCO2 concentration (ANOVA, p = 0.089; Table S6, Fig. 2c). The effect of pCO2 on total dry weight was dependent on food level on day 5 (ANOVA, pCO2 ×food, p = 0.001), but not day 9 (ANOVA, p = 0.448) or day 11 (ANOVA, p = 0.782; Table S4–S6). The significant interaction on day 5 is likely from the particularly high weights in the ambient pCO2/medium food level treatment.
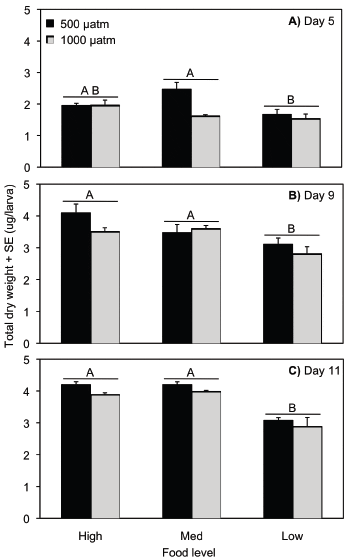
Percent metamorphosis
Settlement occurred on day 16 in the high and medium food treatments in both pCO2 concentrations, on day 19 in the low food/control pCO2 treatment, and on day 22 in the low food/elevated pCO2 treatment. On average, across all food treatments, percent metamorphosis was 70 per cent higher in control compared to high pCO2 concentrations (ANOVA, p < 0.0001; Table S7, Fig. 3). There was also a significant effect of food level on per cent metamorphosis, which was reduced in the low compared to the medium and high food levels (ANOVA, p = 0.01; Table S7, Fig. 3). Percent metamorphosis was 40 per cent higher in the control pCO2/high food compared to the control pCO2/low food treatments, and 54 per cent higher for the high pCO2/high food compared to the high pCO2/low food treatments. The effect of pCO2 level on settlement was consistent across the three food levels (ANOVA, pCO2 ×food, p = 0.226; Table S7).
Discussion
Ocean acidification-induced reductions in growth and calcification have been reported across all life stages of bivalve species (e.g., Ries et al., 2009; Miller et al., 2009; Waldbusser et al., 2010; Gaylord et al., 2011; Hettinger et al., 2012; Gazeau et al., 2013). Several mechanisms are thought to drive such responses including altered metabolic rates and modified energy budget allocation (Pörtner et al., 2004; Michaelidis et al., 2005; Wood et al., 2008; Melzner et al., 2011; Waldbusser et al., 2013). However, studies explicitly testing whether increased food supply might counteract the negative effects of elevated pCO2 focus primarily on adult or juvenile life stages (e.g., Holcomb et al., 2010 (adult corals); Edmunds, 2011 (adult corals); Melzner et al., 2011 (adult mussels); Comeau et al., 2013 (adult corals); Thomsen et al., 2013 (juvenile mussels)). Our limited knowledge of the possible interactive effects of food supply and ocean acidification on larval life stages hampers our ability to predict consequences of global change for natural populations, especially in areas where oceanographic processes create seasonally dynamic conditions of waters that can be depleted in phytoplankton and low in pH.
Eastern boundary upwelling ecosystems (areas bathed by the California, Benguela, Humboldt, and Canary Currents) are among the most productive regions in the world’s oceans, and their response to ocean acidification is therefore of considerable interest. As discussed above, strong upwelling brings nutrient and pCO2-rich water to shallow nearshore environments (Feely et al., 2008; Kudela et al., 2008). Plentiful nutrients fuel rapid population growth of phytoplankton, which operate as a food source for larvae while simultaneously drawing down pCO2 concentrations (Kudela et al., 2008). The timescale of the draw down can also be important: in the initial stages of upwelling, phytoplankton communities may still be in the early stages of population growth, and may not have yet reached high abundance. Under such conditions, low pH may accompany low food supply for larvae, whereas in days hence, the opposite pattern may arise. Thus, during the strong upwelling season along the northern California coast, which typically occurs from May–August, limited food might often accompany high pCO2/low pH conditions, and vice versa. Moreover, upwelled waters have been documented to enter the mouth of bays and estuaries, including those in Oregon and California (e.g., Smith and Hollibaugh, 1997; Barton et al., 2012; A. D. Russell, unpublished data). In these instances, even species such as oysters, whose larvae spend much of their planktonic existence within restricted bodies of water, can experience variable combinations of food and pH.
Whether due to temporally varying upwelling phenomena or other processes, many organisms experience temporary periods of stress and/or reduced nutrition during early development with consequent effects on succeeding performance and life history trajectories (Phillips and Gaines, 2002). Our study revealed that low food supply and elevated pCO2 can each lead to decreased oyster larval growth, weight, and percent metamorphosis. However, the effect of elevated pCO2 concentrations on each response did not depend upon the food supply. Conversely, we found that a benign food environment (i.e., one in which food is not limiting) can partially ameliorate the negative effects of larval exposure to high pCO2 conditions. Melzner et al. (2011) found similar results of high pCO2 and reduced food levels on adult mussels. In this case, shell growth was lower in the high pCO2 and low food treatments, but the effect of pCO2 on shell growth did not depend upon the food level. Thomsen et al. (2013) investigated the effects of food supply and ocean acidification on an earlier life stage of the same mussel species, and juvenile mussels were able to overcome exposure to high pCO2 in high resource environments. The response of juvenile mussels to these interacting stressors could conceivably change if individuals were exposed to varying resources and high pCO2 during the larval stage.
Previous work demonstrated that larval carry-over effects (also referred to as latent effects; Pechenik, 2006) resulted in a magnified, negative response in juvenile Olympia oysters, and effects persisted well into juvenile life (Hettinger et al., 2012, 2013).
A similar trend was exhibited in this study with high pCO2 having a much larger effect on percent metamorphosis (70 per cent) than larval growth (5–14 per cent). The detected reduction in percent metamorphosis might have resulted from larvae starting the energetically expensive process of metamorphosis with depleted energy (i.e., protein and lipid) reserves. Carry-over effects often arise through energetic deficits accrued in prior life history phases. By the completion of the larval phase, oysters exposed to acidified conditions may reach metamorphosis in an energy-depleted state.
Many life processes incur metabolic costs during the larval life phase, and because metamorphosis is also an energetically demanding process (e.g., Videla et al., 1998), any energetic deficits accumulated during the larval phase could be magnified in subsequent life phases. The magnitude of carry-over effects might depend in part on the mode of larval development, especially dependence on exogenous versus endogenous food supply. Olympia oysters are a brooding species that rely on external resources during larval development. Planktotrophic species such as this might be more strongly impacted by changes in phytoplankton abundance and quality compared to lecithotrophic species that are provided with a source of nutrition upon larval release. However, since the maternal environment has been shown to influence offspring performance (Marshall, 2008), any stress from high pCO2 conditions or altered food supply might equally impact planktotrophic and lecithotrophic species directly or indirectly through maternal effects. Early life stages often act as population bottlenecks (Gosselin and Qian, 1997); thus, adult demographics can be influenced by larval carry-over effects that persist into juvenile and adult life.
Our results suggest that Olympia oyster larvae do not demonstrate the ability to fully counteract exposure to elevated pCO2 conditions in high food environments. Furthermore, low food environments and elevated pCO2 conditions both lead to reduced larval growth and total weights, but these stressors operate additively rather than synergistically. This study shows that even when food supply is abundant, larvae exposed to elevated pCO2 are smaller and weigh less than larvae in ambient conditions. This difference in larval size and weight will likely influence juvenile growth through larval carry-over effects, and could have significant implications for adult populations.
March 2014




