Salmonids are indigenous carnivores, and fish meal and fish oil have traditionally been the main ingredients in feed for farmed Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss). Due to limited sources of marine raw materials, a fast growing aquaculture industry and increased focus on sustainability, the salmon farming industry has searched for alternative feeds. The inclusion of plant-derived materials in fish feed may be beneficial from an economic and ecological point of view, but used without carefully considering the fish minimum requirements or upper tolerable levels of certain nutrients or anti-nutrients, it can unfortunately also cause adverse effects with regard to fish health, nutritive value and the consumers’ acceptance.
In the early years of salmonid farming, proteins constituted more than half of the feed content, while the lipid fraction was as low as 10 per cent. These figures have changed during the last decades as the protein level has decreased to less than 40 per cent, while the lipid fraction has increased till about 35 per cent. Fish oil production requires 2-5 times more industrial fish than production of the same weight of fish meal, and replacement of fish oil with vegetable oils is hence of growing interest both from an economic and sustainability viewpoint.
Vegetable oils may contain high levels of n-6 polyunsaturated fatty acids (PUFAs) such as linoleic acid (LA, 18:2n-6) that can be further metabolized to arachidonic acid (AA; 20:4n-6). In contrast, fish oils are rich in n-3 PUFAs such as docosahexaenoic acid (DHA, 22:6n-3) and eicosapentaenoic acid (EPA, 20:5n-3) (Figure 1). In mammals, dietary n-3 fatty acids supplant the AA in inflammatory cell membranes and therefore decrease the availability of the major precursor of pro-inflammatory eicosanoids as the same enzymes are involved in the metabolism of n-3 and n-6 fatty acids and further in the synthesis of eicosanoids. Also in Atlantic salmon, dietary lipid has been shown to alter leucocyte phospholipid fatty acid composition and eicosanoid production, and increased n-6 levels in the feed gave increased n-6 fatty acids in leucocytes in an ex vivo study.
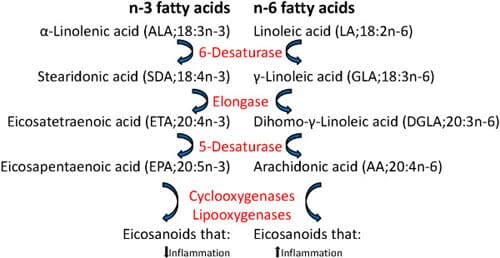
In mammals, a diet rich in n-6 fatty acids has been associated with increased risk of ulcerative colitis and promotion of intestinal carcinogenesis, while a high intake of n-3 PUFAs is considered to be beneficial for health. Consumption of n-3 fatty acids has been shown to attenuate the dysbiosis and colitis caused by n-6 polyunsaturated fatty acid in mice and to prevent and modulate a wide range of pathological conditions as cardiovascular diseases, diabetes and several inflammatory and neoplastic processes, including inflammatory bowel disease and colon cancer. The n-3 fatty acids also inhibit the prostaglandin synthesizing enzyme cyclooxygenase-2 (COX-2) which is up-regulated during inflammation, the expression of the pro-inflammatory cytokines tumour necrosis factor-α (TNF-α) and interleukin-1 (IL-1) and the proliferation of lymphocytes as shown both in vitro and in rodent models.
Several studies have addressed the effects of vegetable oils as lipid sources in the feed on Atlantic salmon intestinal absorption, post-absorptive fates, feed uptake, growth rate, metabolism and nutrient content of the fish filet. Whereas many studies have addressed the intestinal health of the fish when fish meal is replaced by different plant-derived proteins, and both soybean meal and pea protein concentrate have been shown to induce enteritis, there is a knowledge gap regarding the impact on intestinal health when fish oil is replaced by plant oils.
Complete substitution of fish oil with a plant oil blend containing rapeseed oil, palm oil and linseed oil in the feed induced lower transcription levels of certain stress and antioxidant-related genes in the intestine. Another feed trial with the same oil blend partly substituting fish oil in combination with plant proteins at different inclusion levels demonstrated that in response to acute physiological stress, high levels of plant-derived dietary ingredients can enhance COX-2 induction and synthesis of pro-inflammatory eicosanoids in the intestine of salmon. It has also been speculated whether inclusion of plant oils in the feed contributed to intestinal carcinogenesis in brood stock Atlantic salmon.
The aim of the present study was to investigate the morphology of the intestinal wall, the presence of antigen presenting cells and T lymphocytes, the proliferation pattern of epithelial cells, and the transcript levels of selected immune-related genes including relevant cytokines, major histocompatibility complex class II (MHC class II), cluster of differentiation 3ζ (CD3ζ), immunoglobulins, the intracellular receptor nucleotide-binding oligomerization domain-containing protein 2 (NOD2) and COX-2a in the intestine of Atlantic salmon when dietary fish oil was partially replaced by different vegetable oil blends with varying n-3/n-6-ratio.
Results
Histology and immunohistochemistry
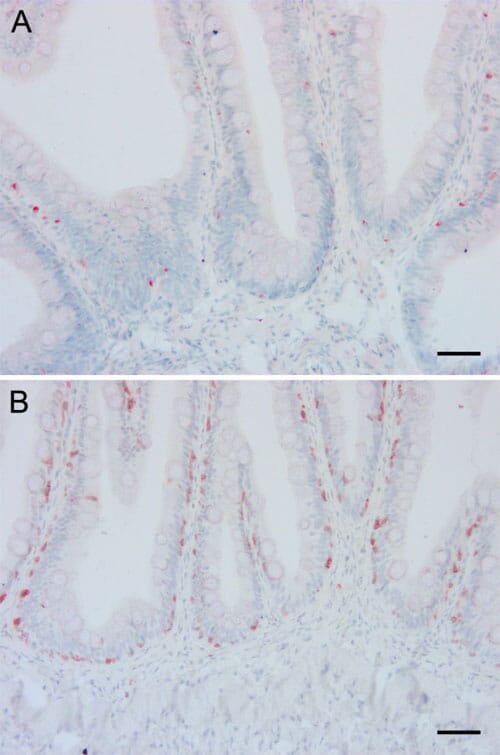
Histological examination of the mid intestine did not show any obvious pathological changes in any fish, while there was shortening, widening and fusion of the simple folds with leucocyte infiltration in the lamina propria in the distal intestine in a few individuals without any obvious association with the different dietary groups. Examination of sections stained with antibodies against MHC class II and CD3? showed positive cells both in the epithelium and in the lamina propria and with similar density and distribution of antigen presenting cells and T lymphocytes regardless of diet (Figure 5). The proliferation pattern of epithelial cells in the mid intestine as demonstrated by immunohistochemical staining with an antibody against PCNA did not differ between the dietary groups.
Morphometric analysis
Mid intestine: The folds of the mid intestine were tallest in the fish oil group (1393 ± 36.4 μm), intermediate in the olive oil and rapeseed oil groups (1134 ± 31.2 μm and 1131 ± 42.4 μm respectively) and lowest in the soybean oil group (1012 ± 24.8 μm). The differences were highly significant (P < 0.0001) between the fish oil group and all vegetable oils groups. The folds of the mid intestine were widest in the soybean oil group (142.0 ± 5.4 μm) and most slender in the olive oil group (122.6 ± 4.3 μm) (P = 0.0249). There was no significant difference between the dietary groups regarding the wall thickness. The results from the morphometric analyses of the mid intestine are shown in Table 2.
Distal intestine: The complex folds were tallest in the rapeseed oil group (3598 ± 100.2 μm) and lowest in the soybean oil group (3123 ± 79.7 μm) (P = 0.0113), while the wall was thickest in the fish oil group (705.2 ± 20.4 μm) and thinnest in the olive oil group (616.8 ± 22.8 μm) (P = 0.0080). There was no significant difference between the dietary groups regarding the height of the simple folds or the width of either simple or complex folds. The results from the morphometric analyses of the distal intestine are shown in Table 2.
Transcript levels of immune-related genes
Only small differences in the relative transcript levels of the various genes between the dietary groups were detected, and there was generally larger individual variation within a group than between the groups. Neither the transcript levels of the pro-inflammatory cytokines IL-1β and TNF-α, the intracellular receptor NOD2 nor the enzyme COX-2a that is involved in the synthesis of prostaglandins from fatty acids were significantly altered in any dietary group in any of the intestinal segments.
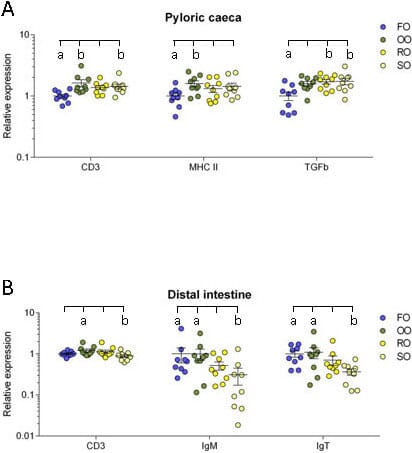
Pyloric caeca: In the pyloric caeca, the olive oil group had significantly higher transcript levels of CD3ζ and MHC class II (P = 0.004 and 0.048) while the rapeseed oil group had a significantly higher transcript level of TGF-β (P = 0.022) and the soybean oil group had significantly higher transcript levels of CD3ζ and TGF-β (P = 0.033 and 0.017) compared to the fish oil group (Figure 6A).
Mid intestine: The transcript levels of the selected genes appeared to be more uniform in the mid intestine, 7and no significant differences between any dietary groups were detected in this intestinal segment.
Distal intestine: In the distal intestine, the transcript level of CD3ζ was significantly higher in the olive oil group than the soybean oil group (P = 0.035). The transcript levels of both IgM and IgT was significantly lower in the soybean oil group than in the fish oil group (P = 0.014 and 0.016) and the olive oil group (P = 0.015 and 0.038) (Figure 6B).
The underlying data for Figure 6A and B and the normalized transcript levels for all genes and dietary groups relative to the fish oil group are shown in Table 3. A disadvantage of calculating normalized transcription levels is that the differences between genes get lost, while the absolute Ct-values will reveal such differences. The Ct-values for MHC class II were relatively low (~18-20) in all intestinal segments of the fish in all dietary groups, indicative of a high transcript level of mRNA. The Ct-values for CD3ζ and the immunoglobulins were intermediate (~24-30), while the Ct-values for the selected cytokines, NOD2 and COX-2a were in general high (~31-37) and for some individuals beyond the detection limit. The mean Ct-values for all examined genes in the three intestinal segments for the fish oil group were included in Table 1.
Discussion
In this study, we have shown that fish fed diets where fish oil was largely replaced by three different vegetable oil blends had significantly shorter folds in the mid intestine compared with fish fed a diet with fish oil as the sole lipid source in a trial lasting for 28 weeks. The fold height decreased to a degree roughly corresponding to a decreasing n-3/n-6 fatty acid ratio of the feed, ie. the fish in the soybean oil group had the shortest folds. Additionally, the mid intestinal folds of the fish in the soybean oil group were significantly wider than of the fish in the olive oil group. In the distal intestine, the complex mucosal folds of the fish in the soybean oil group were significantly shorter than the folds of the fish in the rapeseed oil group, while the wall was significantly thicker for the fish in the fish oil group than the fish in the olive oil and soybean oil groups. Histological and immunohistochemical examination did not reveal any overt signs of inflammation in the lamina propria of the mid intestine. In the distal intestine, however, infiltration of inflammatory cells was observed in some individuals, but this could not be related to the dietary groups as fish in the fish oil group also were affected. Real time RT-PCR revealed only minor alterations in the transcript levels of the selected immune-related genes between dietary groups.
Dietary lipid sources have been reported to affect intestinal morphology in mammals. In weaning pigs, dietary supplementation with fish oil increased villus height in the small intestine combined with a decrease in transcript levels of inflammation related genes compared to a diet with corn oil. Furthermore, dietary fatty acid composition has been reported to affect the height of intestinal villi in ileum in rats, the extent of the reductions increasing with increasing levels of n-6 fatty acids, ie. rats fed with fish oil had higher villi than those fed with olive oil and soybean oil. Interestingly, rats fed with soybean oil had wider villi than the group given olive oil but not the group given fish oil, which is in agreement with our observations. The altered morphology was followed by a corresponding infiltration of mucosal lymphocytes.
In Atlantic salmon, shortening and widening of the simple mucosal folds of the distal intestine, in combination with infiltration of inflammatory cells in the lamina propria, has been repeatedly reported when feeding with soybean meal (SBM) and pea protein concentrate. Starvation has also been described to mildly induce similar changes. In humans, shortened intestinal villi and inflammation in the small intestine occurs in patients with coeliac disease caused by reaction to gluten proteins. However, in the present study, histological investigation and immunohistochemical examination with antigen presenting cell and T lymphocyte markers did not show infiltration of inflammatory cells in the intestinal wall corresponding to the fold reduction pattern. Furthermore, there was no significant difference in the transcript levels of the investigated genes between either of the groups in the mid intestinal region. Combined, this indicates that the fold reductions observed in the mid intestinal region in the current study were not connected with a prolonged inflammatory response, but were probably caused by other factors.
In fish, the gastrointestinal microbiota is known to change with different feeding regimes and more specifically with different lipid levels and different vegetable oils. Alterations in intestinal microbiota are hence not to be neglected as a possible explanatory factor for the altered morphology observed.
Significant reductions of mucosal folds in the mid intestine of all vegetable dietary groups were observed, in contrast to the mildly affected distal intestine. This finding might be related to the fact that long chain fatty acids (LCFAs) mainly are absorbed in the pyloric caeca and mid intestine and only to a limited extent in the distal parts of the intestine. Altering the composition of the LCFA in the feed can hence be speculated to cause most pronounced changes in the regions where these fatty acids are mainly absorbed.
Shortening of mucosal folds has been linked to altered proliferation pattern of the intestinal epithelium in Atlantic salmon; decrease in cell proliferation and apoptosis in smolt exposed to sublethal levels of inorganic mercury and increase in cell proliferation in soybean meal induced enteropathy. We did however not observe any differences in the proliferation pattern of epithelial cells in the mid intestine between any of the diet groups, even though lower turn-over in cell proliferation and apoptosis has been previously detected in Atlantic salmon fed with a diet where fish oil was completely replaced by plant oil.
Shortening of the mid intestinal folds probably reduces the total surface of the intestine and hence the absorption of nutrients, which may in turn influence the growth of the fish. A substantial proportion of starch and lipids is absorbed in the mid intestine. Fish fed soybean oil were significantly shorter than the fish fed fish oil and somewhat (but not significantly) lighter than the other fish in the trial; results which were linked to reduced feed intake in the soybean oil group. It cannot, however, be ruled out that the pronounced shortening of the folds in the mid intestine of fish fed soybean oil may be an additional factor contributing to the somewhat reduced growth in fish fed soybean oil. Furthermore, the difference in weight between the groups might have been more pronounced if not the fish fed fish oil as the sole lipid source had significantly reduced lipid digestibility due to high levels of dietary saturated fatty acids. It cannot be ruled out that the reduced lipid digestibility in the fish oil group may have affected intestinal morphology as response to saturated fatty acids possibly being above an acceptable threshold level for Atlantic salmon. Overall, the shortened intestinal folds being most pronounced in the soybean oil group suggest that feeding with similar or higher levels of soybean oil as in the present trial might not be unproblematic for the production results.
In the pyloric caeca, the transcript levels of TGF-β and CD3ζ in the soybean oil group, the transcript level of TGF-β in the rapeseed oil group and the transcript levels of MHC class II and CD3ζ in the olive oil group was significantly higher than in the fish oil group. TGF-β is produced by cells of the innate immune system and by regulatory T lymphocytes and is both a pro- and anti-inflammatory cytokine that is involved in cell growth, migration, differentiation and apoptosis including inhibition of lymphocyte proliferation. In SBM-induced inflammation in Atlantic salmon, transcription levels of TGF-β were reported up-regulated by 7-folds after 21 days, combined with a 20-folds up-regulation of IL-1β. In the present trial, although significant, the differences observed in TGF-β transcript levels in the fish fed soybean oil and rapeseed oil compared to the fish fed fish oil was below two-fold, and there was no significant difference in IL-1β transcript levels between the groups; hence it is difficult to ascribe the differences in TGF-β transcript levels to an inflammatory process. The finding that neither the relative transcript levels of TNF-α, NOD2 nor COX-2a did vary significantly between the dietary groups in any of the intestinal segments also suggests that dietary lipids did not affect the degree of inflammation. The transcript levels of TNF-α and IL-1β in leucocytes from Atlantic salmon did not differ significantly between groups that were incubated in plasma with different n-3/n-6 ratio followed by stimulation with LPS, and a relative similar EPA/AA ratio in the cells was launched as an explanation to the lack of influence of fatty acid sources on inflammatory response. A similar EPA/AA ratio in leucocytes may explain the relative stable transcript levels of cytokines regardless of diets in the current study too.
In contrast to mammals harboring mesenteric lymph nodes and distinct lymphoid follicles in the intestinal mucosa, the immune competent cells including antigen presenting cells as well as T and B lymphocytes are more diffusely spread in the intestinal tissue of teleosts like Atlantic salmon. The moderately higher transcript levels of CD3ζ observed in the pyloric caeca of the olive and soybean oil group might indicate a slightly higher number of T lymphocytes as CD3ζ is part of the T cell receptor complex and expressed in all T lymphocytes. MHC class II is in contrast expressed in antigen presenting cells that can present antigenic peptides to T lymphocytes and initiate the adaptive immunity. In mammals, and presumably in teleosts, MHC class II is moreover expressed in intestinal enterocytes. A higher transcript level of MHC class II might indicate a higher level of antigen presentation in the pyloric caeca of the olive oil group compared to the fish oil group. The differences in both CD3ζ and MHC class II transcript levels between the groups were however below two-fold and should be carefully interpreted. The higher transcript levels of certain genes in the plant oil groups compared to the fish oil group in the pyloric caeca could be linked to the high lipid absorption in this region. However, this does not explain why we do not see a corresponding change in the transcript levels for these genes in the mid intestine as absorption rate for LCFAs are reported to be similarly high here.
The significantly lower transcript level of CD3ζ in the distal intestine of the soybean oil group compared to the olive oil group might in contrast to the pyloric caeca, indicate a lower number of T lymphocytes. Furthermore, the soybean oil group had significantly lower transcript levels of IgM and IgT than the fish fed both fish oil and olive oil. These immunoglobulins are expressed by different subpopulations of B lymphocytes in the teleost intestine and are present both as membrane bound and secretory forms. Again, the differences were moderate, but summed together they suggest a difference in response to soybean oil between the pyloric cecea and distal intestine.
In mammals, replacement of n-3 fatty acids with n-6 polyunsaturated acids has been associated with intestinal inflammation and promotion of intestinal cancer. Feed-induced intestinal carcinogenesis following inflammation has also been reported in brood stock Atlantic salmon, and it was speculated whether this partially could be related to replacement of fish oil with vegetable oils in the feed. Although the brood stock were exposed much longer to the commercial feed than the fish in the current 28 week-long trial, the results of the present study strongly suggest that partial replacement of fish oil with vegetable oils in the feed did not induce prolonged intestinal inflammation in Atlantic salmon.
The amount of fish oil still present in the feed might be of importance for the ability of the fish to cope with the increased amount of n-6 fatty acids. The regular feed composition used in the salmon industry has changed dramatically over the last decades as the lipid fraction has increased from 10 per cent till about 35 per cent. This means that although vegetable oils constitute 80 per cent of the lipid fraction as in the feeds of our study, the feed still contains approximately 1.4 per cent EPA and DHA provided by the fish oil and fish meal included in the feed, which might be enough to sustain general intestinal health. It has been shown previously that 1 per cent EPA and DHA in the feed is essential to attain good growth in fry. A minimum proportion of EPA and DHA is considered to be required also for larger Atlantic salmon, however this has not yet been quantified.
Conclusions
The folds in the mid intestine were significantly shorter in all groups of Atlantic salmon fed vegetable oils compared to the group fed fish oil. In the distal intestine, the complex folds were significantly shorter in the fish fed soybean oil compared to the fish fed rapeseed oil. Histological examination did not reveal clear difference in degree of inflammation related to dietary groups, and this finding was confirmed by real-time RT-PCR that only revealed moderate alterations in the mRNA transcript levels of selected immune-related genes.
The shortening of the intestinal folds was most pronounced in the fish fed soybean oil and might be associated with reduced intestinal surface and impaired nutrient absorption and growth. Kept together with significantly higher transcript levels of TGF-β and CD3ζ in the pyloric caeca and significantly lower transcript levels of IgM and IgT in the distal intestine in the fish fed soybean oil compared to the fish fed fish oil, it can be concluded that inclusion of high levels of soybean oil in the feed for Atlantic salmon should be done with caution.
April 2014





