Proliferative kidney disease (PKD) is an economcally important disease affecting salmonid industries in Europe and North America (El-Matbouli et al. 1992, Hedrick et al. 1993).
The disease is characterised by nephromegaly, splenomegaly, glomerulone -phritis, ascites, exophthalmia, pale gills and darkened skin. PKD severity as well as fish host recovery is depen dent on water temperature (Foott & Hedrick 1987, El-Matbouli & Hoffmann 2002, Okamura et al. 2011).
PKD is caused by Tetracapsuloides bryosalmonae (Anderson et al. 1999, Canning et al. 1999, Feist et al. 2001), which belongs to the phylum Myxozoa, class Malacosporea. The T. bryosalmonae life cycle alternates between an invertebrate host (freshwater bryozoans; Canning et al. 1999, 2002) and a vertebrate host, salmonid fish.
Overtly infected bryo zoans re lease the parasite into the water, and fish are infected through gills (Feist et al. 2001, Grabner & El-Matbouli 2010). Kidney, liver and spleen of the fish host can be affected, but the kidney is the main target organ. In the kidney, T. bryosalmonae undergoes multiplication and differentiation from extra- or pre-sporogonic stages to intra-luminal sporogonic stages (Kent & Hedrick 1986), and infected fish release the spores through the urine (Hedrick et al. 2004).
Although many salmonid species are susceptible to T. bryosalmonae, only a few (e.g. brown trout Salmo trutta, infected with the European strain) can excrete the parasite spores to infect the bryozoans and complete the life cycle (Morris & Adams 2006, Grabner & El-Matbouli 2008). In contrast, rainbow trout Oncorhynchus mykiss do not transmit the infection to the bryozoans (Grabner & El-Matbouli 2008).
Rainbow trout held at lower temperatures obtain resistance to the parasite; similarly, rainbow trout that survive a PKD outbreak acquire immunity against the disease in the following season (Foott & Hedrick 1987, de Kinkelin & Loriot 2001). Infection with T. bryosalmonae seems to play a significant role in declines of wild salmonid populations: e.g. brown trout in Switzerland, and wild Atlantic salmon Salmo salar in the River Åelva in Central Norway (Wahli et al. 2002, Sterud et al. 2007). The endogenic development of T. bryosalmonae in rainbow trout has been well documented (Bettge et al. 2009, Schmidt-Posthaus et al. 2012).
In contrast, few re ports are available on the chronological development of T. bryosalmonae in brown trout (Clifton-Hadley & Feist 1989, Morris & Adams 2008, Grabner & El-Matbouli 2009, Kumar et al. 2013a, Schmidt-Posthaus et al. 2013). Only limited chronological studies on the effect of PKD on wild salmonid populations have been conducted (Feist et al. 2002, Wahli et al. 2007) due to difficulties adapting sampling protocols, presence of predators and or misdiagnosis resulting from secondary infections (Okamura et al. 2011).
Laboratory investigations are easier to conduct, and in this study we aimed to investigate the persistence and infectivity of T. bryosalmonae in chronically infected brown trout at 24, 52, 78 and 104 wk postexposure (wpe) and the ability of these fish to release viable spores infective for specific pathogen free (SPF) F. sultana colonies, under controlled laboratory condition.
Fish Infection
Among fish exposed to the infected bryozoan Fredericella sultana, no mortalities took place in the first 6 wpe and the fish did not show any abnormal behavior. Four fish from the infected group died in the period 6−10 wpe and 2 fish at 14 wpe, due to PKD, and all were injured (especially on the caudal fin) due to cannibalism caused by variance in growth between individuals and competition for feed.
In the treatment group, kidney samples from both routinely dissected fish at 8 wpe and all 6 mortalities tested positive by PCR for Tetracapsuloides bryo salmonae. Internal PKD symptoms including swollen kidney, pale liver and enlarged spleen were obvious in both fish dissected at 8 wpe and in the fish that died at 6−10 wpe. Only moderate exophthalmia and darkened skin were exhibited by all fish in the infected group 12−26 wpe, but these symptoms had disappeared by the end of the experiment.No other clinical signs were observed and no ectoparasites wereseen on gills or skin. The 2 fish sampled from the control group at 8 wpe were PCR-negative for T. bryo salmonae.
Exposure of SPF F. sultana colonies to infected brown trout
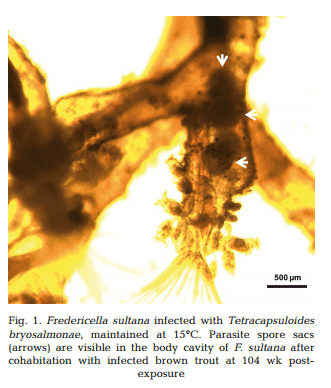
Approximately 4−5 wk after each exposure to infected brown trout (at 26, 52, 78 and 104 wpe), unattached motile overt developmental stages of T. bryosalmonae were observed in the body cavities of exposed F. sultana colonies. Over the following 4 wk, overt infections with characteristic mature sacs were seen floating in the metacoel (Fig. 1). F. sultana zooids were PCR-positive for T. bryosalmonae.
PCR and IHC
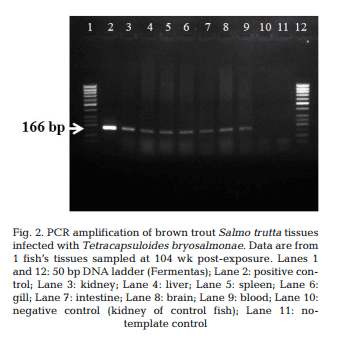
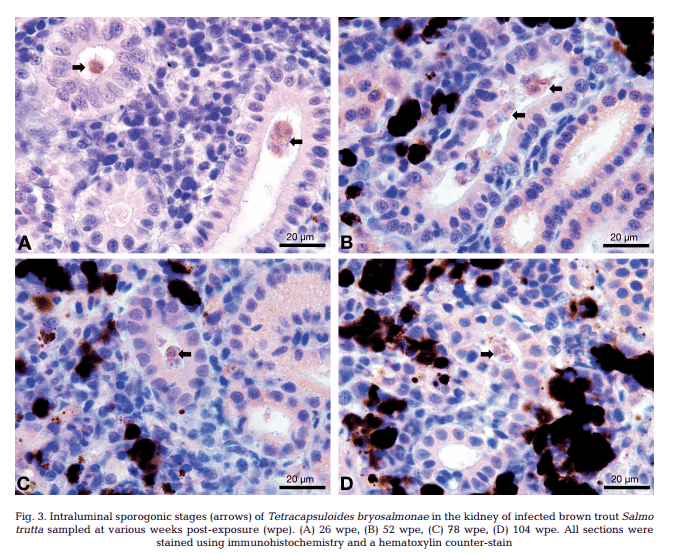
T. bryosalmonae DNA was detected by PCR from kidney, liver, spleen, intestine, brain, gills and blood at 26, 52, 78 and 104 wpe from all fish (Fig. 2). In contrast, blood and organs (kidney, liver, spleen, intestine, brain and gills) from all 20 control fish were negative by PCR. IHC assay of kidney sections from chronically infected brown trout showed intra-luminal sporogonic stages of T. bryosalmonae in the renal tubules and a proliferation of interstitial cells with melanomacrophage centers at all 4 time points (Fig. 3).
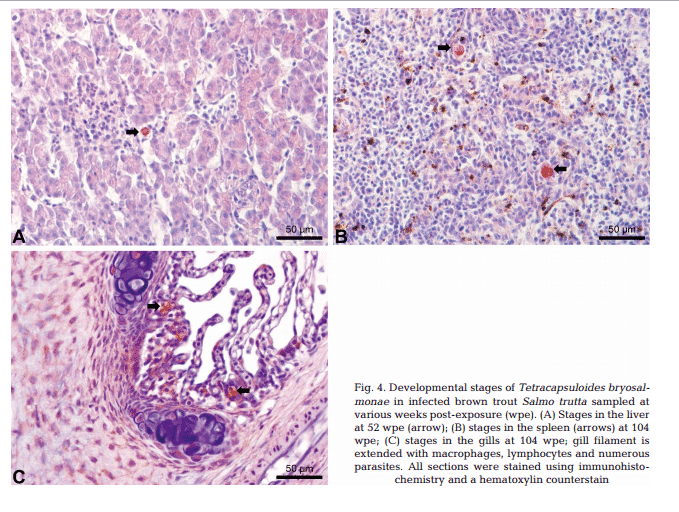
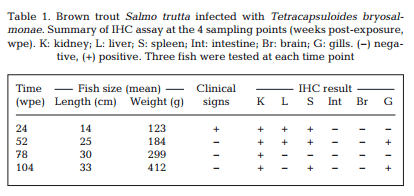
Maturing spores with polar capsules were observed exclusively in renal tubules. Pre-sporogonic parasite stages were detected in the liver at 26 and 52 wpe (Fig. 4A), and spleen at 26, 52 and 104 wpe (Fig. 4B), while pre-sporogonic stages were present in gills at 52 and 104 wpe only (Fig. 4C). No pre-sporogonic stages were observed in the brain and intestine at any time points. The IHC assay results are summarized in Table 1. None of the control fish showed any positive IHC signals of parasite infection (data not shown).
We verified the presence and persistence of Tetracapsuloides bryo salmonae developmental stages in laboratory infected brown trout up to 2 yr from initial exposure and showed that they were still capable of infecting the bryozoan host Fredericella sultana. Clinical signs of PKD, including renal hypertrophy, hepatomegaly and splenomegaly were observed only in fish dissected 6−10 wpe.
Minor to moderate swelling of kidneys and spleen was observed in 12% of infected brown trout up to 52 wpe, which is in accordance with results of Holzer et al. (2006). However, most fish sampled at 26, 52, 78 and 104 wpe did not show any clinical signs. These results were not extraordinary since fish usually do not develop clinical signs at 15°C (Clifton-Hadley etal. 1984, Bettge et al. 2009). Foott & Hedrick (1987) found that rainbow trout that survived PKD infection had sporogonic stages inside their kidney tubules for at least 1 yr following exposure to the parasite.
This finding was supported by Morris et al. (2000), who found T. bryosalmonae sporogonic stages in the kidney tubules of brown trout and Atlantic salmon, outside the PKD season. In the present study, we observed internal clinical signs in fish 6−10 wpe only; however moderate exophthalmia and darkened skin persisted until 24 wpe. T. bryosalmonae DNA were detected by PCR in all kidney samples from each fish at the 4 different time points.
This accords with results of Schmidt-Posthaus et al. (2012), who detected T. bryosalmonae DNA by PCR in rainbow trout up to 29 wpe (201 d, until the end of the experiment). Schmidt-Posthaus et al. (2013) suggest that the persistence of T. bryo samonae DNA may be due to either residual parasite DNA post-infection or latent parasite which might be reactivated. Our cohabitation trials with SPF F. sultana indicated that the parasite remains virulent, with successful transmission to the bryozoan host.
In the present study, IHC assay revealed persistence of sporogonic stages of T. bryosalmonae in the kidney lumen of sampled fish at the 4 time points (26, 52, 78 and 104 wpe). Holzer et al. (2006) observed intravascular developmental stages of T. bryo salmonae in the heart of brown trout by in situ hybridization at 30−45 wpe.
Our results also agree with Schmidt-Posthaus et al. (2013), who show persistence of parasite in the kidney lumen of brown trout exposed naturally to T. bryosalmonae in warm rivers during summer. In contrast to brown trout, laboratory-infected rainbow trout did not show any positive signals by IHC after 15 wpe (103 d), independent of water temperature (Schmidt-Posthaus et al. 2012).
In the same study, Schmidt-Posthaus et al. (2012) comment on the possibility of persistence of viable parasites in kidneys of infected brown trout survivors. Our results indicated that the parasite can indeed remain viable in the host at least up to 104 wpe after initial exposure, but the mechanism by which the parasite maintains itself is still to be elucidated.
Holzer et al. (2006) reported that T. bryo salmonae pre-sporogonic stages are present in the spleen and liver of brown trout at 36 wpe. We demonstrated the persistence of presporogonic stages in multiple organs in brown trout (Table 1) and an apparent inability of the fish immune system to eliminate the parasite. It is well known that PCR is more sensitive than IHC, and this is shown in our study by some tissues being PCR positive but IHC negative (Table 1).
Similarly, Skovgaard & Buchmann (2012) reported that while all samples that tested positive for T. bryosalmonae by IHC were confirmed with PCR, not all PCR-positive samples could be confirmed by IHC.Kent & Hedrick (1986) found PKD developmental stages in the blood vessels and blood smears from infected rainbow trout at 4 wpe, suggesting that the parasite first multiplies in the blood before being distributed to other tissues. We were surprised to detectparasite DNA in the blood by PCR 104 wk after initial exposure.
Existence of blood stages of many myxozoan species has been studied (Dyková & Lom 1988); myxozoans use the blood for proliferation and transport of developmental stages (Björk & Bartholomew 2010, Holzer et al. 2013). Morris & Adams (2004) recognized a distinct blood form of T. bryosalmonae from those in the kidney interstitium, based on the distribution of carbohydrate in their primary cells. Holzer et al. (2006) reported T. bryosalmonae presporogonic stages in the intravascular tissue of the heart. Blood stages of T. bryosalmonae as revealed by in situ hybridization were small (15°C); also Morris et al. (2005) found that 10% of naturally infected rainbow trout held in the laboratory for 36 wk (9 mo) after initial exposure showed clinical signs of PKD at constant water temperature (18°C), while, 90% of infected rainbow trout had recovered.
Holzer et al. (2006) found that kidney samples of Age 1+ brown trout (second year fish kept in a pond system) were PCR-positive for T. bryo salmonae, which indicate that T. bryosalmonae was still present throughout the second summer. Additionally, in situ hybridization revealed stages of T. bryosalmonae in the epithelium and lumen of the renal tubules in Age 1+ brown trout. Morris & Adams (2008), using IHC, found pseudoplasmodia in the kidney tubules of brown trout infected with T. bryo salmonae at constant water temperature (18°C).
We found persistence of T. bryosalmonae sporogonic stages in the kidney tubules of brown trout, suggesting that persistence of parasite depends on the fish species and their immune systems (Kumar et al. 2013a). The above-mentioned results indicate that the parasite can persist for a long period in brown trout regardless of the temperature regime (either at a constant temperature under laboratory conditions, or variable temperature as in a pond system).
Additionally, Gay et al. (2001) reported that T. bryo salmonae can develop in the bryozoan F. sultana at water temperatures of 7−8°C, since the spores were present throughout the year. Therefore, our laboratory temperature 15°C (± 1°C) was optimal for both vertebrate and invertebrate hosts and does not interfere with the pathogenesis and development of the parasite. Schmidt-Posthaus et al. (2012) found that chronically infected rainbow trout (held at 12°C) could completely regenerate renal morphology and eliminate the majority of the parasite. Kumar et al. (2013a) demonstrated similar results in rainbow trout at 16.5°C at 17 wpe using IHC and quantitative RT-PCR (qRT-PCR).
The only difference between these 2 studies was the temperature, which appears to in fluence the rate but not the progress of recovery (Ferguson 1981, Clifton-Hadley et al. 1986, Schmidt-Posthaus et al. 2012). In contrast to rainbow trout, our results from brown trout show that this species is unable to completely clear the infection after 104 wpe, while held at constant temperature and the absence of any source of re-infection.
We also demonstrated that recovered brown trout were able to infect the bryozoan host up to 104 wpe. This finding suggests that brown trout which survive infection with T. bryosalmonae may act as carriers of the parasite. Schmidt et al. (1999) attributed the decline of brown trout catches in Switzerland to PKD, which was supported by Wahli et al. (2002, 2007). Wahli et al. (2002) surveyed wild brown trout and rainbow trout, and found disease prevalence ranged between 40 and 100% at some Swiss river sites.
Recently, we reported stages of T. bryosalmonae in the statoblasts (dormant stage) of F. sultana, which indicates not only the occurrence of vertical transmission but also the possibility that the parasite can persist in the bryozoan host and be distributed to new locations (Abd-Elfattah et al. 2014).
Our current results demonstrate persistence of T. bryosalmonae also in the fish host and their ability to infect F. sultana colonies up to 24 mo from the initial exposure. Taken together, these aspects of the parasite’s biology explain persistence of PKD in endemic waters and farms and its wide geographic distribution. Further studies are needed to address how long the parasite can persist in wild brown trout as well as the role of blood stages in parasite maintenance in the host over long periods.
November 2014