“Early Mortality Syndrome” (EMS), more technically known as acute hepatopancreatic necrosis disease (AHPND), is a new shrimp disease that is presently disrupting production in the major shrimp producing countries China, Thailand, Vietnam and Mexico.
Being caused by specific strains of Vibrio parahaemolyticus that are difficult to eradicate from the production environment, AHPND will require a very different approach than the current strategies against white spot virus (WSSV) based on specific bio-security measures.
Avoiding early contamination through the broodstock and larvae, combined with continued control of the microbial populations particularly during the initial month of the cycle, will be crucial to control EMS.
The use of antibiotics to control microbial developments throughout the production process is not desirable due to the risk for building up resistance and its rejection by legislators and consumers. The shrimp industry requires alternative ways to control the microbial ecosystem in production systems.
Sustainable approaches to modulate the gut microflora in shrimp include the use of a wide variety of natural compounds capable of modulating the microflora towards a favorable composition such as probiotics, organic acids, yeast extracts and phytobiotics.
Synergistic effects between these different compounds is most probable, for example phytobiotics can enhance the establishment of probiotic bacteria and therefore enhance the effects of probiotic inoculations in the production system.
Functional feeds containing gut health promotors deliver with every meal an adequate concentration of natural antimicrobial activities into the shrimp gut. These feeds are a key component of any strategy to prevent EMS.
However, the success of this approach will depend on the efficacy of the selected gut health promotor against the pathogenic bacteria involved in EMS.
The gut modulating feed additive ideally is heat stable and can therefore be easily incorporated into the feed at the feedmill and be present in every meal from the starter feed onwards, without requiring major adaptations of the production protocols at the nursery or farm.
Natural feed additives combining different action mechanisms against Vibrio species such as direct bactericide/bacteriostatic properties as well as Quorum Sensing inhibition properties at concentrations below MIC, are most promising to reduce the impact from EMS.
In the present study, three different types of health promoting feed additives were evaluated on their capability to reduce the effects of an experimental AHPND infection on the build up of Vibrio in the shrimp’s digestive system and the mortality during 2 weeks following the infection.
Trial protocols
The challenge trial was performed at the Minh Phu AquaMekong Shrimp Vet Laboratory (MPA). Juvenile whiteleg shrimp (Penaeus vannamei) were fed with a commercial diet mixed with one of three feed additives for 21 days prior to the challenge with EMS-causing Vibrio parahaemolyticus (VP). During the 15 days of post-challenge follow-up period, shrimp continued to be fed the same diet they received during the pre-challenge period to determine if functional feed additives could confer protection against VP.

Postlarvae 12 days old (SPF, SIS broodstock) were transferred from the hatchery to MPA where they were grown in strict biosecurity for another 45 days to reach the size of 1-2 g. One day prior to the start of the study, SPF P. vannamei at 1-2 gram/each were transferred to 90L tanks. All animals in these tanks were fed their respective test diet for 21 days at approximately 5% bodyweight each day. All aquaria were outfitted with an oyster shell filter, aeration and covered with plastic to reduce the risk of cross contamination.
Three different types of additives with anti-microbial properties were evaluated compared to the un-supplemented control diet: SANACORE, a synergistic blend of natural antimicrobial compounds with multiple actions including bacteriostatic and quorum sensing inhibition; PHYTO, a botanical blend with antimicrobial activity; and OAC, a blend of organic acids.
Challenge methods
MPA in conjunction with the University of Arizona's Aquaculture Pathology Laboratory (UAZ-APL) has developed some standardized challenge methods used to challenge small juvenile shrimp with the strain of Vibrio parahaemolyticus that causes EMS/AHPND simulating the natural routes of infection (via water exposure and cannibalism).
In this study, two challenge methods were used: bathing (immersion) and direct feeding of bacteria (per os).
For the immersion challenge, Tryptic Soy Broth +2% sodium chloride (TSB+) inoculated with a consistently virulent strain of Vibrio parahaemolyticus (strain LA37), incubated for 18 hr at 28 °C, was added directly into tanks to reach a bacterial density of 3.105 cells/ml measured by optical density absorbance (OD600 nm).
For the per os challenge, TSB+ medium inoculated with LA37 and incubated for 18 hr, was mixed with commercial shrimp feed at 20% v/v ratio and air-dried for 15 min. The shrimp feed coated with bacterial culture was fed to shrimp for only one meal at 3% body weight. The negative control tanks were also fed with a commercially pelleted shrimp diet but were treated with sterile TSB+ instead of bacterial suspensions.
A day zero sample consisting of two animals was preserved in Davidson’s AFA fixative prior to the start of the challenge study to document the animals’ SPF health status for WSSV, TSV, EMS/AHPND, and IMNV. Shrimp total Vibrio count on TCBS were done in serial dilutions on day 5, 10 and 15 post-challenge animals of each treatment. Sampled shrimp were peeled, homogenated in saline water, and diluted serially before plating on plates of different media for counting.
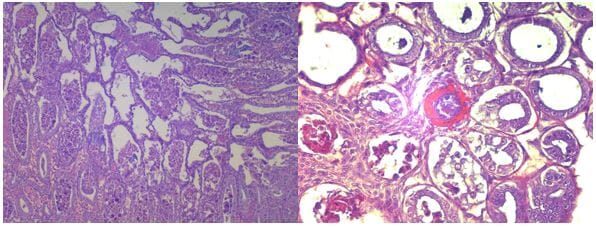
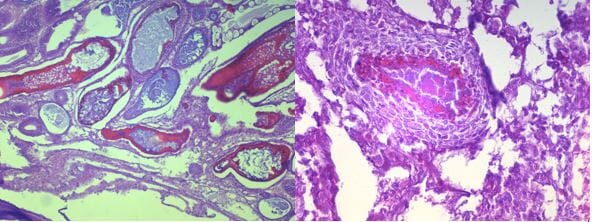
The tanks were checked twice daily, all mortalities were removed from the tank and frozen for later PCR test. On day 21 (just prior to the challenge tests), and on day 5, 10 and 15 post-challenge up to three moribund animals of each treatment were preserved in Davidson’s AFA fixative and processed for routine histology and PCR to confirm the presence of EMS/AHPND pathology.
At termination of the challenge study, all live animals were counted as survivors.
Results and Discussion
The histological analysis shows that shrimp in the negative control groups remained negative to EMS throughout the experiment. Meanwhile, shrimp from all other challenged groups exhibited EMS lesions after challenge.
Interestingly, shrimp in the challenged groups in general showed positive to EMS after 5 days and 10 days of challenge but may not show lesions of EMS after 15 days of challenge. This may indicate that shrimp may have the capability to recover from EMS infection if they still survive the challenge.
The non-challenged, negative control group showed an average survival of 91% which indicated that the shrimp used in the current challenge trial were in a good health and nutritional status throughout the trial.
Following challenge, the mortality occurred gradually during the 15 days of post-challenge for both types of experimental infections, which is due to the selection of a VP strain with medium virulence (Fig. 1).
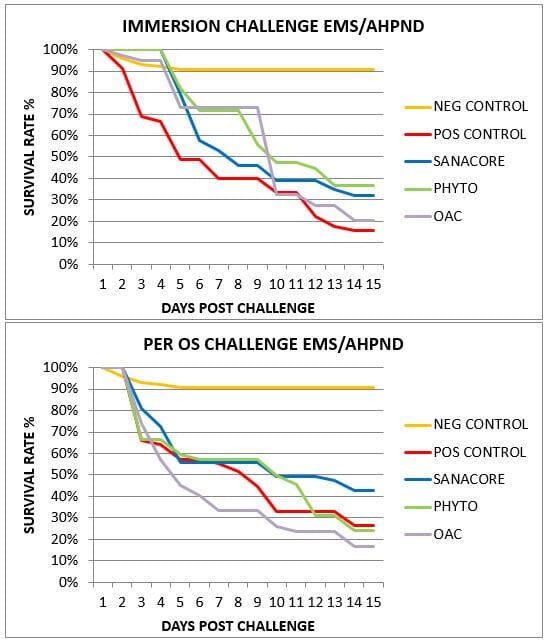
Nevertheless, in the immersion challenge shrimp supplemented with feed additives showed a slower mortality initially compared to control. This was not the case for the more direct per os challenge.
Shrimp fed the control feed (positive control treatment) showed a final survival of 15.6% and 26.7% for the immersion and per os challenge, respectively.
Supplementing the feed with different health additives during 21 days prior to the challenge and following 15 days after the challenge did not result in significant differences in challenge survival compared to control for any of the treatments (average survival 16.7-42.9%; Fig. 2).
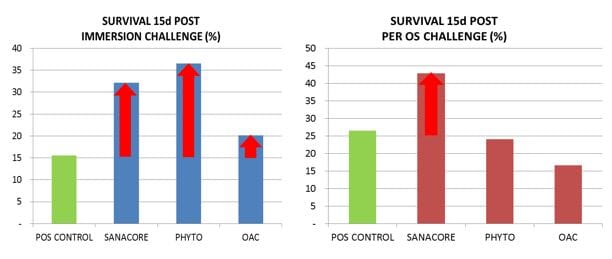
Fig 2: Final survival for positive control group and three treatments fed different health additives, experimentally infected by immersion or per os.
However, survival at the end of the trial indicated some trends which differed according to the two types of challenges (immersion and per os; Fig. 2).
The shrimp supplemented SANACORE tended to show higher survival compared to control in both types of challenges (+107% in the immersion challenge, +62% in the per os challenge). The group fed the botanical mix (PHYTO) showed higher survival only in the immersion challenge (+135%).
The group supplemented with the mix of organic acids (OAC) did not show any effect in any of the two challenges.
The treatment resulting overall in the best challenge survival (Sanacore) exhibited consistently lower plate counts in the shrimp’s digestive system compared to the other treatments, both in total heterotrophic count and total Vibrio count (Fig. 3).
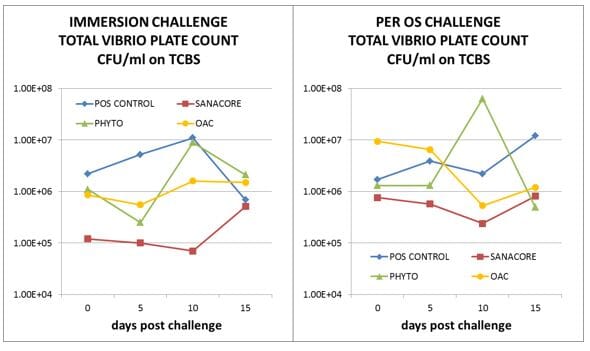
Interesting to note is that the lower total Vibrio count compared to the control group was already established for this treatment following 3 weeks of acclimatization to the diet (day 0 in Fig. 3).
The current lab results using a controlled AHPND infection are consistent with recent results obtained from field trials in Mexico and Vietnam in EMS affected farms. These field data indicated that specific health promoting feed additives bring up survivals from medium levels (60-70%) to pre-EMS levels (>85%).
However, farm results also show that such feed additives are not a magic bullet but rather an essential component of a total farm management strategy aiming at controlling the microbial balance throughout the production cycle.
Authors
From Minh Phu AquaMekong Shrimp Vet Laboratory:
-
Loc H. Tran
-
Phuc Nhu Hoang
-
Oanh Hoang Bui
-
Trang Dai Nguyen
From Nutriad International:
-
Allen Ming-Hsun Wu
-
Sam Ceulemans
-
Peter Coutteau
May 2015