Identity
Cyprinus carpio Linnaeus, 1758 [Cyprinidae]
FAO Names: En - Common carp, Fr - Carpe commune, Es - Carpa

Biological features
Body elongated and somewhat compressed. Lips thick. Two pairs of barbels at angle of mouth, shorter ones on the upper lip. Dorsal fin base long with 17-22 branched rays and a strong, toothed spine in front; dorsal fin outline concave anteriorly. Anal fin with 6-7 soft rays; posterior edge of 3rd dorsal and anal fin spines with sharp spinules. Lateral line with 32 to 38 scales. Pharyngeal teeth 5:5, teeth with flattened crowns. Colour variable, wild carp are brownish-green on the back and upper sides, shading to golden yellow ventrally. The fins are dusky, ventrally with a reddish tinge. Golden carp are bred for ornamental purposes.
View SIDP Species fact sheet
Profile
Historical background
The carp was a luxury food in the middle and late Roman period, and it was consumed during fasting in the middle Ages. The fish were kept in storage ponds ('piscinae') by the Romans, and later in fish ponds constructed by Christian monasteries. In this European practice the carp were kept in monoculture. The largest individuals were selected as broodfish. From, the 12th to the mid-14th century A.D. unintentional artificial selection had taken place, the first steps towards domestication. Controlled semi-natural pond breeding and fry rearing of carp started in the 19th century in Europe. Cyprinids have been reared in China for more than 2 000 years, where they were kept in undrainable ponds. The ponds were stocked regularly with fry from rivers. Natural food-based polycultural rearing technology was applied. Semi-domesticated carp races have developed in this system. Domesticated carps have been produced in most of the carp rearing areas recently. There are about 30-35 strains of domesticated common carps in Europe. Many strains are maintained in China. There are some Indonesian carp strains, which have not been scientifically examined and identified so far.
Habitat and biology
Wild common carp (generally referred to as 'carp' in this fact sheet) live in the middle and lower streams of rivers, in inundated areas, and in shallow confined waters, such as lakes, oxbow lakes, and water reservoirs. Carp are mainly bottom dwellers but search for food in the middle and upper layers of the water body. Typical 'carp ponds' in Europe are shallow, eutrophic ponds with a muddy bottom and dense aquatic vegetation at the dikes.The ecological spectrum of carp is broad. Best growth is obtained when water temperature ranges between 23 °C and 30 °C. The fish can survive cold winter periods. Salinity up to about five per cent is tolerated. The optimal pH range is 6.5-9.0. The species can survive low oxygen concentration (0.3-0.5 mg/litre) as well as supersaturation.
Carp are omnivorous, with a high tendency towards the consumption of animal food, such as water insects, larvae of insects, worms, molluscs, and zooplankton. Zooplankton consumption is dominant in fish ponds where the stocking density is high. Additionally, the carp consumes the stalks, leaves and seeds of aquatic and terrestrial plants, decayed aquatic plants, etc. The pond farming of carp is based on the ability of the species to accept and utilise cereals supplied by the farmers. The daily growth of carp can be 2 to 4 per cent of body weigh. Carps can reach 0.6 to 1.0 kg body weight within one season in the polycultural fish ponds of subtropical/tropical areas. Growth is much slower in the temperate zone: here the fish reach the 1 to 2 kg body weight after 2 to 4 rearing seasons.
In Europe, female carp need about 11 000 to 12 000 degree-days to reach maturity in the temperate and subtropical climatic zones. Male carp are matured within a period that is 25-35 per cent shorter. The maturity period of Asian carp strains is slightly shorter. The spawning of European carp starts when the water temperature is 17-18 °C. Asian strains start to spawn when the ion concentration of the water decreases abruptly at the beginning of the rainy season. Wild carps are partial spawners. Domesticated carps release all their matured eggs within a few hours. After hormonal treatment carp release their ripe eggs within a much shorter period, which makes stripping possible. The quantity of released eggs is 100 to 230 g/kg body weight. The egg shell becomes sticky after contacting water.
The embryonic development of carp takes about 3 days at 20-23 °C (60-70 degree-days). Under natural conditions, hatched fry stick to the substrata. About three days after hatching the posterior part of the swim bladder develops, the larvae swim horizontally, and start to consume external food with a maximum size of 150-180 µm (mainly rotifers).
Production
Production cycle
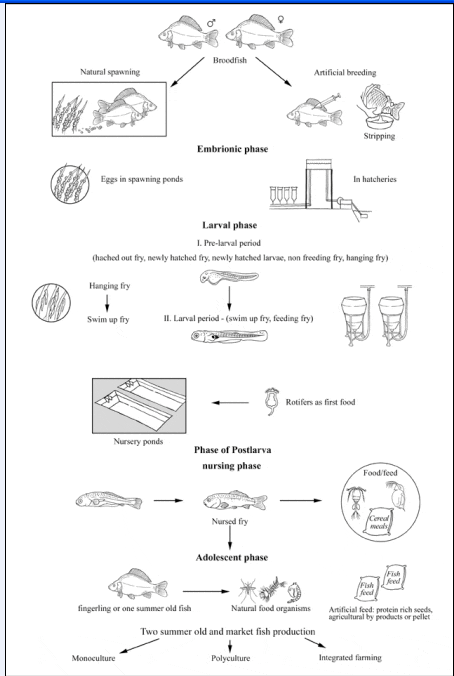
Production systems
Seed supply
Spawning on nests, aquatic weeds and inundated grass in tanks and ponds
Carp may spawn throughout the year in tropical areas of India, with peaks in January-March and July-August. Breeding is carried out in hapas, cement tanks or small ponds. Submerged aquatic plants are used as substrata for egg laying. When the fry are 4 to 5 days old, they are stocked into nursery ponds.
The 'Sundanese method' is used for spawning carp in Indonesia. The broodfish are kept in broodfish ponds, segregated by sex. Matured broodfish are transferred to 25-30 m² spawning ponds. 'Kakabans' (nests made of the fibre of Arenga species) are installed into the ponds. The fish lay their eggs on both sides of the kakabans. When spawning is completed, the nests are transferred to hatching/nursing ponds.
Small ponds are used for spawning carp in China. Aquatic weeds (Ceratophyllum, Myriophyllum) or floating palm leaves are used as spawning substrata.
Small 'Dubits ponds' (120-300 m² water surface area) were used for spawning, and for short nursing of carp fry in Europe in the past. More recently, ponds with an area from a few hundred m² up to 10-30 ha are used here. Two to four weeks after spawning, the fry can either be harvested from these large ponds, or may remain there up for rearing to fingerling size.
Hatchery based seed production
This is the most effective and reliable method of seed production. Broodfish are kept in water saturated with oxygen, within the temperature range of 20-24 ºC. They are given two doses of pituitary gland injection, or a mixture of GnRH/dopamine antagonist, to induce ovulation and spermiation. The eggs are fertilized (applying the 'dry method') and the adhesiveness of the eggs is eliminated using salt/urea treatment, followed by a tannin acid bath (the 'Woynarovich method'). Incubation is carried out in Zoug jars. The hatched fry are kept in large conical tanks for 1 to 3 days, and are usually stocked at the stage of 'swim-up' or 'feeding fry' into properly prepared ponds. Approximately 300 000 to 800 000 newly hatched fry can be expected from a single female.
Nursery
Nursing of common carp in ponds and tanks
Shallow, aquatic weed-free drainable ponds of 0.5 to 1.0 ha are the most suitable for carp nursing. Nursery ponds must be prepared before stocking to encourage the development of a rotifer population, since this constitutes the first food of feeding fry. The stocking density is 100-400 fry/m². The ponds should be inoculated with Moina or Daphnia after stocking. Supplementary feeds, such as soybean meal, cereals meals, meat meal, or mixtures of these materials, should be applied. Rice bran or rice polishings can also be used for feeding fry. The length of the nursery period is 3 to 4 weeks. The final fish weight is 0.2-0.5 g. The survival rate is 40-70 per cent.
If there are many predators in the area where ponds would be situated (insects, snakes, frogs, birds, wild fish), tank nursing of carp can be applied. Tanks of 5-100 m² surface area, made of concrete, bricks or plastic, can be used for nursing fry up to 1-2 cm in size. By applying hay and manure, dense populations of Paramecium and rotifers can be established in these tanks. A few hundred fry per m² can be stocked. Collected zooplankton and fine particle size meals, or complete starter foods can be used. Industrial type systems, such as raceways, or water recirculating systems are also suitable for nursing.
Fingerling production
The production of carp fingerlings normally takes place in semi-intensive ponds, based on manure/fertilizer-generated natural food and supplementary feeding. Fingerling production can be carried out in a single stage system (stocking newly hatched fry and harvesting fingerlings), a dual stage system (stocking nursed fry and harvesting fingerlings), or a multicycle system (when newly hatched fry are stocked, and the fish are thinned out several times).
Stocking nursed fry is the most effective way for producing medium and large size fingerlings. Depending on the required final size of fingerlings, 50 000-200 000 nursed fry/ha can be stocked in temperate zones, preferably in polycultural systems where the proportion of common carp is 20-50 per cent. The final weight of the carp is 30-100 g. In warm climates, if large size fingerlings are the production target, the stocking density of nursed fry is 50 000-70 000/ha, out of which the proportion of common carp is 20 per cent. Survival rates of 40-50 per cent are achieved. Small size fingerlings can be produced in ponds stocked with 400 000 small (15 mm) nursed fry. In this case the survival rate is 25-30 per cent.
Frequent application of manure is necessary to maintain the plankton population. The feeding is based mainly on agricultural by-products in subtropical areas, on cereals and/or pellets in temperate zones.
Ongrowing techniques
Production of two summer-old carps
In temperate zones, one-summer old fish (20-100 g) must be reared up to 250-400 g in the second year. The stocking rate is 4 000-6 000/ha, plus about 3 000 Chinese carp/ha, if only cereals are fed. The stocking rate can be much higher (up to 20 000/ha) if cereals and pellets also used. The daily ration is approximately 3-5 per cent of body weight.
Production of market size fish
Common carp can be produced in extensive, natural food and supplementary feed-based monocultural production systems, in stagnant water ponds. Artificial feed-based intensive monocultural production can be carried out in cages, irrigation reservoirs, and running water ponds and tanks, or in recirculation systems.
Common carp are stocked with Chinese carps, and/or Indian major carps, tilapia, mullet, etc., in polycultural systems. This constitutes a natural food and supplementary feed-based production method, in which fish that have different feeding habits and occupy different trophic niches are stocked into the same ponds. The quantity of fish should be in accordance with the productivity of natural food organisms. The frequent application of manure or fertilizers and the proper species ratio, make the maintenance of productive populations of natural food organisms, and the maximal utilization of the productivity of pond ecosystem possible. Synergetic effects between fish species support the production in polycultural ponds.
Carp culture can be integrated with animal husbandry and/or plant production. Integration can be direct (animals above fish ponds), indirect (wastes of animals are used in the ponds as manure), parallel (rice-cum-fish), or sequential (fish production between crops). The sequential cycling of fish/animal/legumes/rice (in 7 to 9 year cycles) is suitable for significantly decreasing the environmental loading of intensive aquaculture/agriculture. Since common carp burrow in the pond bottom, have a broad environmental tolerance and an omnivorous feeding habit, they are a key species in integrated systems.
Common carp can also be stocked into natural waters, reservoirs, and temporarily inundated areas, in order to utilize the natural food production of these waters for enhanced capture fisheries. In this case the fish stocked should be 13-15 cm fingerlings produced in fish farms ('aquaculture-based fisheries') in order to avoid the losses that would occur with smaller fish. Common carp are usually stocked with other cyprinid species, in accordance with the productivity of the water and the intensity of exploitation.
Feed supply
The use of natural food has been mentioned in other sections of this fact sheet. These are sometimes supplemented with compounded farm-made or commercial feeds.
Harvesting techniques
Undrainable ponds, or drainable ponds with a long harvesting ditch, or ponds with inner or outer harvesting pits are used for carp rearing. The fish are usually harvested by seine nets. The length of nets should be 1.5 times the width of ponds, but not longer than 120-150 m.
In undrainable ponds, selective harvesting can be done. The maximum weight of carp which can get through various mesh size nets are: 20 mm mesh size = 20 g fish; 25 mm = 40 g; 30 mm =100 g; 35 mm =170 g; 40 mm = 270 g; 50 mm = 400 g.
Since the carp keep mud-free the area where they search for food, feeding should be done throughout the growing period in the harvest area. At harvest time the water should be drained slowly (1-3 days from a 1 ha pond, 8-14 days from 30-60 ha ponds). The fish gather in the deepest area of the pond, unless they are frightened away by an abrupt decrease of water level, or by noises. Since carp tend to swim towards incoming water, a small quantity of water is flowed into the pond near the drainage site to concentrate the fish, especially if the water temperature is high. When a large quantity of fish is concentrated in the harvesting pits aeration should be supplied. Sprinkling water on the surface is usually not sufficient.
Partial harvesting (regardless whether the ponds are drainable or undrainable) increases the total production of the ponds by improving the conditions for the remaining population.
Handling and processing
If harvesting is carried out in warm water, the fish are pre-conditioned by repeated stressing before netting. Harvested fish can be transferred live in aerated tanks for 3-5 hours, if the fish/water ratio is not more than 1:2. The density of fish in transport tanks and the duration of transport depend on fish size, temperature and the amount of aeration.
If, during harvesting, fish have been enticed into the harvesting area by feed, only very short transport time is feasible, since the oxygen demand of satiated fish is high.
The majority of carps is transferred live to markets, and is sold either live or freshly dressed. Successful trials have been carried out on the large scale filleting of carp in France. Apart from value-added products, about 15 different products can be prepared from carp, representing different levels of processing.
Production costs
The average profit of carp production in some Hungarian fish farms was EUR 326/ha (from sales of EUR 1 652/ha) between 1999-2001, according to a survey by the Research Institute of Fisheries, Aquaculture and Irrigation (unpublished data). In India the net profit from polyculture, in which common carp represented 25 per cent of the total fish stocked, was reported to be USD 710/ha (from sales of USD 1 929) in 1990 (Sinha,1990). The profit of small scale farmers in Bangladesh was reported to be USD 510-1 580/ha (from sales of USD 1 540-2 610/ha) from undrainable polyculture ponds, in which the stocking ratio of carp was 20 per cent (Gupta et al., 1999).
Diseases and control measures
| DISEASE | AGENT | TYPE | SYNDROME | MEASURES |
|---|---|---|---|---|
| Saprolegniosis | Saprolegnia spp. | Fungus | White fungal colonies on body surface, wounded areas or ulcers, and on egg surface | Single or repeated doses of malachite green |
| Branchyomycosis; gill rot | Branchiomyces sanguinis | Fungus | Mosaic type coloration of gills; haemorrhages and anaemic areas; mass mortality; secondary Saprolegnia infection | Pond treatment with quick lime; repeated treatment with copper sulphate |
| Carp erythrodermatitis; ulcer disease | Aeromonas salmonicida achromogenes | Bacterium | Small spherical nodules on fins; haemorrhages; ulcers with jagged rims; protruding scales; exophthalmia; swollen abdomen; haemorrhages on gills; pinkish fluid in the body cavity; secondary Saprolegnia infection of ulcers | Apply extensive technologies; avoid stress; apply antibiotics in feed or as injections; vaccination |
| Columnaris disease | Flexibacter columnaris | Bacterium | Appearance above 15 ºC; grey-white spots surrounded by zone with reddish tinge on the head, gills, skin and fins; destroyed membranes between fin rays | Treatment with benzalkonium chloride, copper sulphate or antibiotics (furazolidone, neomycin, oxytetracycline, terramycin); feed containing sulphamerazine and oxytetracycline |
| Bacterial gill disease | Flavobacterium branciophyla | Bacterium | White areas on the body surface and/or on the gills; necrosis of infected areas | Treatment with salt or antibiotics; improvement of pond environment |
| Mycobacteriosis | Mycobacterium spp. | Bacterium | Emaciated, stunted fish; feeding ceases; light grey discoloration on body surface; sometimes open ulcers | No treatment available; destroy infected populations |
| Spring viraemia of carp | Rhabdovirus carpio | Virus | Outbreak above 12 ºC; erratic swimming; later lethargy occurs; enteritis; oedema; exophthalmia; pale gills; haemorrhages in skin | Elimination of vectors, such as blood sucking parasites; no transfer of infected fish |
| Carp pox | Herpes type virus | Virus | Smooth, opaque, greyish-white patches of 1-2 mm diameter on body surface; later, body is covered with them; loss of calcium; soft body; tail can be turned to head; manifestation above 14 ºC | Avoid introduction of infected fish |
| Koi Herpes Virus Disease (KHV) | Herpes type virus | Virus | Disease occurs between 17-25 ºC on common and koi carp; lethargy; uncontrolled, erratic swimming; focal necrosis of gills; increased mucus secretion; haemorrhages on gills and liver; inflammation of kidney; mass mortality | Keep infected areas free of carp for 3 months; vaccination |
| Costiosis | Ichthyobodo spp. | Protozoan ectoparasite | Gulping at inlet; lethargy; flashing; erratic swimming; slim fish; blue-grey film on skin and gills | Salt, formalin or malachite green baths; copper oxycloride in ponds |
| Coccidiosis | Eimeria spp. | Protozoan endoparasite | Fish lay on pond bottom; hollow eyes; debilitation; thin body; large head; oedema of abdominal membranes and intestinal wall; intestinal wall is dark; swollen intestinal mucosa; yellowish mucus exuded | Disinfection and drying of ponds; Furazolidone in feed |
| White spot disease Ichthyophthiriosis | Ichthyophthirius multifiliis | Protozoan ectoparasite | Scratching behaviour; flashing; gulping; increased gill beat rate; gill damage; white spots on fins, skin, gills and eyes | Malachite green baths |
| Chilodonellosis | Chilodonella spp. | Protozoan ectoparasite | Fish at surface; erratic, flickering swimming; pale gills; grey film of mucus on skin; epithelial cell necrosis; ulcers | Salt, formalin or malachite green baths; copper oxychloride in ponds |
| Trichodinosis | Trichodina spp. | Protozoan ectoparasite | Surfacing; white patches on skin surface; excess mucus exudate on gills; tattered fins; pale gills covered with mucus and cell debris | Salt, formalin or malachite green baths; copper oxycloride in ponds |
| Myxobolosis | Myxobolus spp. | Myxozoan endoparasite | Oedema; loose scales; exophthalmia; white or yellow cysts and haemorrhages on gills; white nodules on gills (koi); muscle necrosis | Fumagilin in feed |
| Dactylogyrosis | Dactylogyrus spp. | Monogenean ectoparasite | Fish swim to water inlet; proliferation of gill epidermis; flukes visible on gills with low (40-60) multiplication | Fumagilin in feed |
| Gyrodactylosis | Gyrodactylus spp. | Monogenean ectoparasite | Fish swimming restlessly; greyish skin; pale gills; white and tattered fins | Salt, ammonia, organophosphate, Neguvon, or praziquantel baths; drying ponds |
| Diplostomosis | Diplostomum spp. | Endoparasitic trematode | Uncontrolled swimming; dark skin; small haemorrhages on abdomen; loss of weight; cataracts develop in eyes; haemorrhages in eyes; inflammation of eyes; exophthalmia | Praziquantel bath; eradication of hosts, such as snails and birds |
| Phosthodiplostomosis | Phosthodiplostomum spp. | Endoparasitic trematode | Encapsulated larvae evoke accumulation of melanina; black cysts of 0.6-1.0 mm develop; deformation of vertebral column may occur in fry | Organophosphate (Masoten, Dipterex, Sumithion) or praziquantel baths; eradication of snails and herons |
| Sanguinicoliasis | Sanguinicola spp. | Endoparasitic trematode | Lethargy; swimming in spiral movement; feeding ceases; fish on water surface; sometimes exophthalmia; gill inflammation | Praziquantel bath; eradication of snails with copper sulphate when no fish present; sun-drying ponds |
| Ligulosis | Ligula intestinalis | Endoparasitic cestode | Distended body; swimming with difficulty; feeding ceases; loss of weight; first part of abdomen bulging; exudum in body cavity; tapeworms visible in fish | Expel birds; praziquantel bath |
| Bothriocephalosis | Bothriocephalus acheilognathi | Endoparasitic cestode | Sluggish movement; swimming at the surface; emaciation; enlarged abdomen; inflammation of digestive tract; haemorrhages and ulcers in gut | Chlorinated salicylanalid in feed; praziquantel bath; keep ponds dry in winter; disinfect pond bottoms with lime; eradicate copepods |
| Khawiosis; tapeworm infestation | Khawia sinensis | Endoparasitic cestode | Sluggish movement; loss of appetite; slow growth; anaemia of skin and gills; haemorrhages and ulcers on gut; worms may protrude from anus | Devermin bath; eradicate tubifex (host) by pond disinfection |
| Nematode infestation | Contracaecum spp. | Endoparasitic nematode | Emaciation; exophthalmia; loss of blood into body cavity; roundworms in heart and body cavity | No treatment |
| Phylometrosis; nematode infestation | Phylometra spp. | Endoparasitic nematode | Lost balance; fish floating head down; feeding ceases; red nodules on skin and under scales | Eradicate copepods; injections of Nilverm or Ditrazin into body cavity |
| Fish Leech infestation | Piscicolidae | Ectoparasitic annelid | Hyperactive swimming at water inlet; loss of weight; ulcers | Salt or Dipterex (with or without potassium permanganate) baths |
| Fish Leech infestation | Piscicolidae | Ectoparasitic annelid | Hyperactive swimming at water inlet; loss of weight; ulcers | Salt or Dipterex (with or without potassium permanganate) baths |
| Ergasilosis | Ergasilus spp. | Ectoparasitic arthropod | Weight loss; slow development; mortality; small white patches on gills; gill hyperplasia; necrosis of gill tissues; lost lamellae; reduced circulation; secondary infections | Chlorfos or organophosphate baths; sun-drying ponds |
| Lernaeosis | Lernaea spp. | Ectoparasitic arthropod | Lethargy; feeding ceases; anchor worms can be seen on body surface and gills | Salt, potassium permanganate or organophosphate baths |
| Argulosis | Argulus spp. | Ectoparasitic arthropod | Parasites visible on body surface; abnormal swimming; lethargy; feeding ceases; excessive mucus production; small haemorrhages; fin erosion; anaemia; ulcers; secondary infections | Salt, potassium permanganate or organophosphate baths |
Statistics
Production statistics
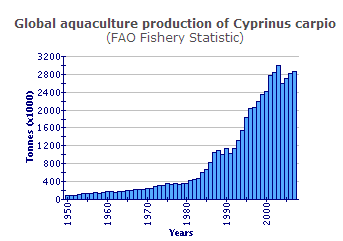
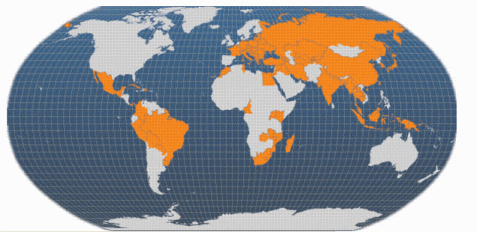
Farmed common carp production was nearly 14 per cent of the total global freshwater aquaculture production in 2002 (33 138 962 tonnes). Common carp production increased by an average global rate of 9.5 per cent/yr between 1985 (681 319 tonnes) and 2002. In the past decade (1993-2002) this has increased to 10.4 per cent/yr. This is greater than the expansion rate of farmed grass carp (10.1 per cent/yr), silver carp (8.8 per cent/yr), and bighead carp production (7.2 per cent/yr), but less than that for tilapias (11.8 per cent/yr) during this decade. In Europe, common carp production was 144 602 tonnes in 2002. This represents a substantial reduction from peak production of over 402 000 tonnes in 1990, caused by changes in Eastern Europe. However, European production appears to be gradually increasing again; the 1993-2002 trough was 125 274 tonnes in 1997.
According to FAO data, the global average unit price of farmed common carp has declined from USD 1.43/kg in 1993 to USD 0.92/kg in 2002. However, this may principally be due to a fall in the value of the RMB yuan during this period in China, where a large proportion of production (e.g. 70 per cent in 2002) takes place.
Market and trade
Statistical data indicate that common carp production may have come close to its limit. However, common carp will remain an important species in those areas where it is produced traditionally. The majority of the carp are consumed domestically. Based on several trials on common carp processing carried out in Europe, it was revealed that live or freshly dressed fish are required by the market. Processing increased the price of carp to less competitive levels, so a significant increase in the demand for processed carp products can not be forecast.
Typically, about 24 000 tonnes of live, fresh/chilled filleted or frozen carp products (all species) are traded (imported or exported) within Europe annually. The main exporters are Austria, the Czech Republic, Croatia and Lithuania. The main importers in 2002 were Austria, Germany, Hungary and Poland. In the whole of the rest of the world, including the principal producing region (Asia), international trading of all carp species is quite limited (39 000 tonnes/yr in 2002).
Production of 'bio carp' has been started in some areas. Quality labelling and an emphasis on the fact that the carp are produced in extensive or semi-intensive systems that are environment-friendly technologies, may increase the acceptance of common carp by certain groups of consumers.
A change in the main objective of common carp production can be observed in Europe. Formerly, the market demanded fish mainly for consumption. Recently, a significant quantity of the carp produced in aquaculture is stocked into natural waters and water reservoirs for angling purposes. Since the anglers prefer fish that are more active on the hook than the domesticated carp, they need wild carp or hybrids of domesticated and wild carp strains. Wild carp are required also for re-stocking natural waters, where the rehabilitation of natural fauna is carried out.
Status and trends
Since this species has outstanding importance in freshwater aquaculture, many aspects of its physiology, nutrition, genetics, and diseases have been studied during past decades. The role of common carp in water ecosystems has been examined, and breeding and rearing technologies that fit various climatic conditions and intensity levels have been developed.
The tasks for the future include:
-
Rearing technology: introduction/adaptation of technologies that are optimal for various climatic, environmental and socio-economic conditions, and the wider application of environmental friendly bicultural and polycultural systems in traditional carp-producing areas.
-
Rotational aquaculture and agriculture: introduction of the rotational use of land for agricultural/carp-based aquacultural systems may help to eliminate the adverse environmental impact of intensive agriculture in many places. This system can also be used for soil desalination.
-
Genetics: practice-oriented genetic research needs to be continued for the development of reliable breeding systems. Based on genetic research, breeding associations should be established for maintaining the stabile 'landraces' (strains) in various geographical areas and climatic zones, in order to avoid inbreeding. INGA (International Network on Genetics in Aquaculture, organised by the World Fish Center, formerly ICLARM) helps to fulfil the above tasks in Southeast Asian and East European areas. There is some scope in fish genetics for increasing the disease resistance of carps by the development of resistant strains and hybrids.
-
Diseases and control: adverse changes in the natural environment, the increasing intensity of carp production in many areas, extensive inter-regional transport of common carp and other cyprinids, and the ban on using several traditional medicaments (fungicides, antibiotics and insecticides) call for the intensification of research on carp diseases. A relatively new and promising field of research is the development of immunostimulants, for increasing the natural resistance of fish. The development of vaccines seems to be the most promising solution for avoiding the application of antibiotics. Development and large-scale application of vaccines against viral diseases have primary importance to control 'traditional' viral diseases, such as the spring viremia, carp pox and viral gill necrosis. Large-scale introduction of vaccination against 'KHV' (which is actually a virus called Carp Nephritis and Gill Necrosis Virus, CNGNV) is also very important in the infected or endangered areas. The development of rapid diagnostic tools to determine the bacterial and viral infections is also necessary. Vigilance on parasitical diseases should be maintained. Research on better understanding of pre-conditioning environmental and technological factors, which make the fish less resistant and the pathogens more virulent, should also be continued.
Main issues
The effect of extensive carp farming on the environment is negligible or even positive, since the carp help to maintain aerobic bottom conditions. The environmental effect of semi-intensive polycultural carp farming depends on the intensity of production, and on the water quality of recipients. The accumulation of silt and organic material can be very high in integrated systems. However, the rotational use of land for fish-cum-duck and alfalfa and rice production is the most environmental friendly means of conducting aquaculture and agriculture. The effect of intensive (industrial) aquaculture systems on the environment depends on the efficiency of waste management.
The overstocking of open waters with carp and the introduction of non-indigenous carps may cause negative impacts. The population of aquatic weeds can be destroyed by increasing turbidity and uprooting plants. By decreasing the spawning grounds available for phytophil species, common carp may decrease the biodiversity in natural waters.
Responsible aquaculture practices
There are many well elaborated types of carp production, so it is relatively easy to select production methods that accord with Article 9 of the Code of Conduct for Responsible Fisheries. The most widely applied technique, namely supplementary feed-based extensive or semi-intensive carp production, is considered as an environmentally friendly way of animal protein production. Responsible aquaculture on the production level (Article 9.4., Code of Conduct) can be ensured by applying a strict process of licensing, in which the main principles of environment and ecological protection are considered.
The establishment of carp breeding associations that maintain and breed pure strains of common carp by certified breeders in licensed fish hatcheries; frequent quality control based on standardized progeny testing; and supporting farms in the stocking of pure strains, helps to maintain the carp population of various areas, including the wild-type carp populations of natural waters this system was elaborated and applied by the Association of Hungarian Fish Producers.
Fish health control based on local veterinarians and government institutions helps to increase the security of production by decreasing the effects of the diseases of farmed fish on the natural fish population, and helping to minimize the use of chemicals, drugs and antibiotics.
The introduction of quality controls, based on the labelling/traceability of the products, and the provision of support for the development of 'organic' products may increase the application of environmentally friendly technologies, as well as improving the supply of good quality fish.
January 2010




