Identity
Macrobrachium rosenbergii De Man, 1879 [Palaemonidae]
FAO Names: En - Giant river prawn, Fr - Bouquet géant, Es - Langostino de río
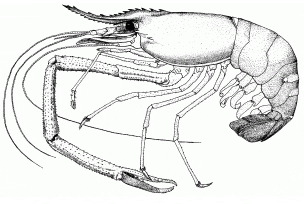
Biological features
Males can reach total length of 320 mm; females 250 mm. Body usually greenish to brownish grey, sometimes more bluish, darker in larger specimens. Antennae often blue; chelipeds blue or orange. 14 somites within cephalothorax covered by large dorsal shield (carapace); carapace smooth and hard.
Rostrum long, normally reaching beyond antennal scale, slender and somewhat sigmoid; distal part curved somewhat upward; 11-14 dorsal and 8-10 ventral teeth. Cephalon contains eyes, antennulae, antennae, mandibles, maxillulae, and maxillae. Eyes stalked, except in first larval stage. Thorax contains three pairs of maxillipeds, used as mouthparts, and five pairs of pereiopods (true legs). First two pairs of pereiopods chelate; each pair of chelipeds equal in size. Second chelipeds bear numerous spinules; robust; slender; may be excessively long; mobile finger covered with dense, though rather short pubescence. Abdomen has 6 somites, each with pair of ventral pleopods (swimmerets). Swimmerets of sixth abdominal somite stiff and hard and, with the median telson, serve as the tailfan. Eleven distinct larval stages.
Images gallery




Profile
Historical background
Although reared in captivity from time immemorial, modern farming of this species originated in the early 1960s when FAO expert Shao-Wen Ling, working in Malaysia, found that freshwater prawn (Macrobrachium rosenbergii) larvae required brackish conditions for survival. This discovery led to larval rearing on an experimental basis. By 1972 the Hawaiian team led by Takuji Fujimura had developed mass rearing techniques for commercial-scale hatchery production of prawn postlarvae (PL). This development spawned the first commercial farms in Hawaii and elsewhere. Both Thailand and Taiwan Province of China became pioneers in modern giant river prawn culture.
The introduction of broodstock, initially from Hawaii and Thailand, into non-indigenous areas around the world began in the 1970s. The first major FAO project designed to expand the culture of this species began in 1978 in Thailand. Since then, giant river prawn culture has developed in every continent, particularly in Asia and the Americas. Global production had increased to over 200 000 tonnes/yr by 2002 (including production in Viet Nam). Furthermore, there is considerable production of other freshwater prawn species, notably M. nipponense that does not appear in official statistics.
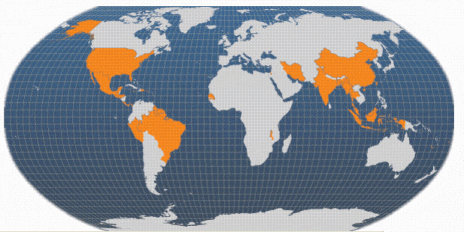
Note: Viet Nam is also a major producer of farmed M. rosenbergii; this is not illustrated on this map because Viet Nam includes its production within the category 'freshwater prawns, shrimps nei' (not elsewhere included) when making data returns to FAO.
Habitat and biology
This species lives in tropical freshwater environments influenced by adjacent brackishwater areas. It is often found in extremely turbid conditions. Gravid females migrate downstream into estuaries, where eggs hatch as free-swimming larvae in brackishwater.
Before metamorphosis into postlarvae (PL), the planktonic larvae pass through several zoeal stages. After metamorphosis, PL assume a more benthic life style and begin to migrate upstream towards freshwater. Larvae swim actively tail first, ventral side uppermost. From PL onwards prawns swim forwards, dorsal side uppermost.
From metamorphosis onwards prawns can also walk, not only on the sub-stratum but also over damp areas including stones by river edges, up vertical surfaces (small waterfalls, weirs, etc.) and across land. Larvae mostly consume zooplankton (mainly minute crustaceans), very small worms, and larval stages of other crustaceans.
Postlarvae and adults are omnivorous, eating algae, aquatic plants, molluscs, aquatic insects, worms, and other crustaceans. Males and females have different growth rates and males exhibit heterogenous individual growth (HIG); these are vitally important factors in grow-out management. Three distinct male morphotypes (and a number of intermediary types) exist: small male (SM), orange claw males (OC), and blue claw males (BC). The normal male developmental pathway is SM to OC to BC. BC males have extremely long second pereiopods; those of OC males are golden coloured; SM have small, slim, almost translucent claws. The type and behaviour of the males affects the growth rates of other prawns. The transition from rapidly growing OC to the slowly growing BC morphotype follows a "leapfrog" growth pattern. An OC metamorphoses into a BC only after it has become larger than the largest BC in its vicinity. The presence of this new BC male then delays the transition of the next OC to the BC morphotype, causing it to attain a larger size following its metamorphosis. BC males dominate OC males, regardless of their size, and suppress the growth of SM.
Production
Production cycle
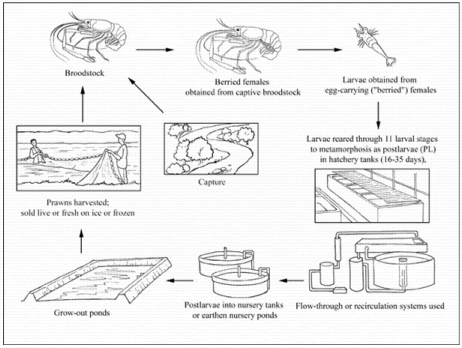
Production systems
Seed supply
When required for hatchery use, female broodstock are usually obtained from grow-out ponds but also sometimes from capture fisheries. Normally, "berried" (egg-carrying) females are only used once. Commercial farms in tropical regions do not normally maintain captive broodstock for breeding purposes but adults are over-wintered indoors in temperate regions in order to stock ponds with PL as early as possible in the short grow-out season. The typical male to female ratio in broodstock holding systems is 1-2 BC males or 2-3 OC males per 20 females, at a total stocking density of 1 prawn per 40 litres. Within a few hours of copulation, fertilisation occurs externally, as the eggs are transferred to the brood chamber beneath the abdomen. The eggs remain adhered to the female during embryonic development, which lasts about 3 weeks. At hatching, free-swimming zoeae are produced. Between 5 000 and 100 000 eggs are carried, depending on the size of the berried female. Eggs are orange until 2-3 days before hatching, when they become grey-black.
Some seed (PL; juveniles) is obtained from the capture fishery where M. rosenbergii is indigenous, typically in the Indian sub-continent, but most is now hatchery-reared. First stage zoeae are just under 2 mm long and grow, through 11 larval stages, to almost 8 mm at metamorphosis into PL. Individual metamorphosis can be achieved in as little as 16 days but usually takes much longer, depending on environmental conditions. In commercial hatcheries, most larvae metamorphose by day 32-35 at the optimum temperature (28-31 °C). Larval rearing typically occurs in 12‰ brackishwater, and hatcheries are either flow-through (where a proportion of the rearing water is regularly replaced) or recirculating (where a variety of systems involving physical and biological filters are used to minimise water use).
Either type of hatchery may be inland or coastal. Inland hatcheries produce brackishwater by mixing freshwater with seawater transported from the coast, brine trucked from salt pans, or artificial seawater. Some flow-through hatcheries use a "greenwater" system, which involves fertilisation to encourage the growth of phytoplankton (mainly Chlorella spp.), which is believed to improve water quality and increase larval survival; others operate a "clearwater" regime. Feeding systems vary widely but typically include brine shrimp (Artemia salina) fed several times per day at first, reducing to a single daily feed by larval stage 10. Prepared feed (usually an egg custard containing mussel or fish flesh, squid, or other ingredients) is introduced at stage 3 and its feeding frequency is increased towards metamorphosis. Some hatcheries are integrated with nursery and grow-out facilities.
Nursery
Although some farmers stock grow-out ponds with young PL, many either purchase larger juveniles or rear PL in their own nursery ponds before transfer to grow-out ponds. In temperate areas with a limited grow-out season, environmentally controlled indoor nurseries are used to increase animal size before stocking outdoors as soon as temperatures become high enough. Indoor nurseries are stocked at 1 000-2 000 PL/m³, depending on whether substrates are used or not. Outdoor nurseries may be stocked with newly metamorphosed PL or with juveniles from an indoor nursery. Typically, stocking rates are 1 000/m² PL, 200/m² small juveniles (0.02 g) or 75/m² of 0.3-0.4 g juveniles, but increased densities are possible if substrates are used.
Ongrowing techniques
Freshwater prawns are reared in a variety of freshwater enclosures, including tanks, irrigation ditches, cages, pens, reservoirs, and natural waters; the commonest form being earthen ponds. Normal rearing methods comprise various combinations of the formerly used "continuous" (ponds operated indefinitely, with regular cull-harvesting and restocking) and "batch" (single stocking, single harvesting) systems; these are known as "combined systems". Most systems involve monoculture, but the polyculture of freshwater prawns with finfish and sometimes other crustaceans also occurs, particularly in China (with carps). Integration of freshwater prawn culture with crop production also occurs (typically in Viet Nam).
Pond stocking densities in tropical monoculture vary widely. In extensive rearing systems (typically producing <500 kg/ha/yr), PL or young juveniles are stocked at 1-4/m²; semi-intensive systems (producing 500-5 000 kg/ha/yr) are stocked at 4-20 PL or young juveniles/m². Rarely, some small intensive systems also exist, which stock >20/m² to achieve >5 000 kg/ha/yr. In temperate areas with a limited rearing window of opportunity about 5-10 PL/m² or 4 juveniles/m² are stocked; levels can be increased in presence of substrates.
The prawns are fed commercial or "farm-made" feeds, the latter being single or mixtures of ingredients, often extruded through mincers and either fed moist or (usually) after sun-drying. Feeds with 5 percent lipid and 30-35 per cent protein are commonplace and an FCR of 2:1 or 3:1 is achieved with dry diets. Average growth rates depend on many factors, particularly the way in which male HIG is managed. The growth rate of SM is stunted by the presence of BC males; in their absence SM metamorphose into OC and ultimately into BC males. Thus the way in which grow-out ponds are managed (for example, the frequency of culling out large prawns, mostly males) influences total productivity.
Harvesting techniques
Harvesting is either total (in "batch" rearing) or partial (in "continuous" or "combined" rearing). Total harvesting is achieved by gravity drain-down or water removal through pumping, while seine nets are used for regularly culling larger animals. Stretched knot mesh sizes of 1.8 cm are use to harvest small prawns and from 3.8-5.0 cm for large prawns. The time and frequency of harvesting depends entirely on the volume and characteristics (the animal size) of market demand.
Handling and processing
Careful handling is essential from harvesting onwards to ensure good quality products. Freshwater prawns tend to go "mushy" if not handled and processed correctly. Firstly, it is essential to prevent prawns from becoming crushed during harvesting. Secondly, if they are not going to be sold live, they should be killed in a mixture of water and ice at 0 °C immediately (at the pond bank), and washed in chlorinated tap water. Prawns for live sale can be transported in aerated water at 20-22 °C. Prawns sold fresh must not be kept on ice for more than 3 days. Prawns for sale frozen must be quick-frozen at -10 °C (not simply placed in a "domestic" freezer) and stored at -20 °C or below.
Production costs
Providing production cost data is difficult, not only because the information is usually proprietary but also because it is site-specific. For example, one review of hatchery operating costs (all figures were normalised to 1997 USD) gave an average of USD 10.6/1 000 PL but the range was USD 1.1 to 17.0/1 000 PL. Hatchery investment costs ranged from USD 12.4-19.0 per 1 000 PL production capacity. Nursery investment costs for producing 4 million 2 g juveniles in Latin America, for example, has been quoted as USD 50 000. Nursery operating costs have been quoted as USD 6 per thousand 2 g juveniles and USD 50 per thousand 10 g juveniles in Thailand. Typically, feed expenses represented at least 40 per cent of total nursery production costs. Investment costs for grow-out facilities have been cited as ranging from USD 400/ha (yielding 600 kg/ha/yr) in India to nearly USD 64 000/ha (yielding 2 270 kg/ha/yr) in the USA. Similarly, grow-out operating costs from less than USD 2 to more than USD 15/kg/yr have been cited. The average cost split was 30 percent feeding, 20 percent seed, 15 percent labour and 35 percent other expenses.
Diseases and control measures
The major disease problems affecting Macrobrachium rosenbergii generally occur because of poor intake water treatment, poor husbandry, overcrowding, poor sanitation, and non-existent or inadequate quarantine procedures. The measures to combat these problems are referred to as improved husbandry (IH) in the table below, which records some of the more important diseases. In some cases antibiotics and other pharmaceuticals have been used in treatment but their inclusion in this table does not imply a recommendation.
| DISEASE | AGENT | TYPE | SYNDROME | MEASURES |
|---|---|---|---|---|
| MMV (Macrobrachium Muscle Virus) | Parvo-like virus | Virus | Infected tissue becomes opaque, with progressive necrosis; affects juveniles | IH |
| WSBV (White spot Syndrome BaculoVirus) | Baculovirus | Virus | White spots; affects juveniles | IH |
| Unnamed viral disease | Nodavirus | Virus | Whitish tail; affects larvae | IH |
| Black spot; brown spot; shell disease | Vibrio; Pseudomonas; Aeromonas | Bacteria | Melanised lesions; affects all life stages, but more frequently observed in juveniles & adults | IH; oxolinic acid; nifurpurinol |
| Bacterial necrosis | Pseudomonas; Leucothrix | Bacteria | Similar to black spot but only affects larvae, especially stages IV & V | IH; nifurpurinol; erythromycin; penicillin-streptomycin; chloramphenicol |
| Luminescent larval syndrome | Vibrio harveyi | Bacterium | Moribund & dead larvae luminescent | IH; chloramphenicol; furazolidone |
| White postlarval disease | Rickettsia | Bacterium | White larvae, especially stages IV and V | IH; oxytetracycline; furazolidone; lime, prior to stocking |
| Unnamed fungal infection | Lagenidium | Fungus | Extensive mythelial network visible through exoskeleton of larvae | IH; trifluralin; merthiolate |
| Unnamed fungal infection (often associated with IMN - see below) | Fusarium solani | Fungus | Secondary infection; affects adults | IH |
| Unnamed yeast infections | Debaryomyces hansenii; Metschnikowia bicuspidata | Fungi | Yellowish, greyish or bluish muscle tissues in juveniles | IH |
| Protozoan infestations | Zoothamnium; Epistylis; Vorticella; Opercularia; Vaginicola; Acineta; Podophyra; etc. | Protozoans | External parasites that inhibit swimming, feeding and moulting; affect all life stages | IH; formalin; merthiolate; copper-based algicides |
| IMN (Idiopathic Muscle Necrosis) | environmental disease | Unknown | Whitish colour in striated tissue of tail and appendages; when advanced, necrotic areas may become reddish; affects all life stages | IH |
| MCD (Mid-Cycle Disease) | undetermined aetiology | Unknown | Lethargy; spiralling swimming; reduced feeding and growth; bluish-grey body colour; affects larvae, especially stages VI and VII | IH; hatchery disinfection |
| EED (Exuvia Entrapment Disease), sometimes known as MDS (Moult Death Syndrome) | undetermined aetiology | Unknown but probably multiple causes, including nutritional deficiency | Localised deformities; failure to complete moulting; affects late larval stages; also seen in postlarvae, juveniles & adults | IH; dietary enrichment |
Statistics
Production statistics
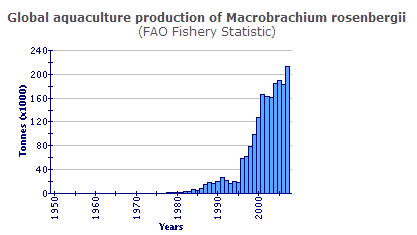
The global production data shown above do not include the considerable production from Viet Nam, which is a component of the category 'freshwater prawns, shrimps nei'. Global production in 2002 exceeded 200 000 tonnes.
Market and trade
Both domestic and international markets exist and are expanding. Peeled, mostly wild-caught Macrobrachium rosenbergii have long been exported globally, but farmed shell-on (and normally head-off) freshwater prawns are also a familiar sight in the supermarkets of Europe now. To a lesser extent, this also occurs in the USA (mainly for consumption by Asians or in restaurants serving Asian food) and Japan. India, Bangladesh, Viet Nam and Thailand export a significant proportion of their wild-caught and farmed prawns. Potential for expansion exists but small-scale producers may need to co-operate in collective marketing to exploit these opportunities. Freshwater prawns are a distinct product from marine shrimp, which have their own favourable culinary characteristics.
Status and trends
Farmed production is rapidly expanding in Asia. The rate of expansion in the largest producer, China, has slowed, partly due to the farming of an alternative indigenous species (Macrobrachium nipponense) and partly because marine shrimp are now being reared in freshwater (and are sometimes referred to erroneously as freshwater prawns). Chinese production actually fell in 2002 but, as the global market expands, is expected to expand again later. Production in India and Thailand expanded by more than 50 per cent per year between 1999 and 2002; this trend is expected to continue. There is also potential for expansion in Bangladesh, a traditional exporter from its capture fisheries. Indonesian production is reported in 2002 for the first time. Viet Nam is a significant producer and exporter of farmed Macrobrachium, although its output is masked by being included in the statistical category 'freshwater prawns, shrimps nei'. Statistically, Brazil also appears as a major producer (>4 000 tonnes/yr since 2000) but this is believed to be an error.
In total, the output of M. rosenbergii from aquaculture expanded during the decade 1993-2002 from 17 000 tonnes to 195 000 tonnes, an APR of 31 per cent/yr. Since 1996, Chinese production has formed a large proportion (58 per cent in 2002); however, even if it is excluded it becomes clear that production elsewhere grew from 17 000 tonnes in 1993 to nearly 82 000 tonnes in 2002, an APR of 19 per cent/yr. Furthermore, from 1999 to 2002, expansion outside China (41 per cent/yr) was much faster than in China (13 per cent).
Further global expansion is difficult to predict, since it depends mainly on the volume of consumer demand. However, even if a very modest expansion of 10 per cent/yr occurs, global farmed production of M. rosenbergii will have significantly exceeded 400 000 tonnes by 2010. The farming of other species of Macrobrachium, notably M. nipponense (already very substantial in China), M. malcolmsonii and M. amazonicum, is also expected to expand.
Main issues
The development of freshwater prawn farming was inhibited in the past by its longer hatchery phase and lower grow-out productivity compared to marine shrimp. These constraints are now balanced by a number of positive factors concerning its sustainability (see responsible aquaculture practices below) and the development of a distinct and expanding market niche for freshwater prawns. Furthermore, fewer poor quality products enter the international markets now that the technique for avoiding "mushiness" has become well-known.
Responsible aquaculture practices
The culture of Macrobrachium spp. is less likely to have a detrimental impact because freshwater prawns cannot be reared at densities as high as those commonly used in marine shrimp farming. Productivity is generally lower, management is less labour intensive, and the potential for the abuse or waste of resources is minimal, and (unlike the inland culture of marine shrimp) the grow-out of Macrobrachium does not make agricultural land saline. Specific negative effects of M. rosenbergii culture on the environment have yet to be documented. Adherence to the FAO Code of Conduct for Responsible Fisheries would ensure that it remains sustainable and responsible. It is particularly well-suited to small long-term family businesses, can be practised by relatively unskilled fishing and rural people, generates products which may be consumed by all social classes, and is amenable to integration with crop production.
April 2010




