Introduction
However, they have become a serious concern only since salmonids have been reared in increasingly large numbers at commercial production facilities1,2. The expansion of salmon and trout aquaculture in Europe, Canada and Chile has been accompanied by increasing infestations of sea lice.
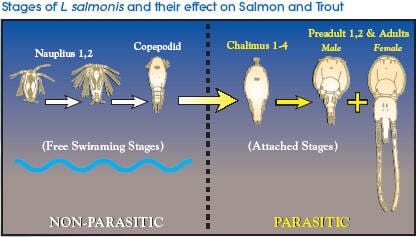
Sea lice feed on fish skin, mucus and blood especially on the head, back and perianal region. Untreated infestations may lead to death from severe erosion and exposure of subcutaneous tissues, secondary bacterial infections, osmotic imbalance and extreme stress. SLICE is a feed premix containing the avermectin, emamectin benzoate, in a 0.2% formulation for the control of sea lice (Lepeophtheirus salmonis and Caligus spp.) on salmon and trout. Emamectin benzoate administered to salmonids in feed at a dose rate of 50 g/kg/day for seven consecutive days kills all parasitic stages of sea lice, i.e. chalimus, pre-adults, and adults including gravid females.
Key Characteristics:
- Administered in feed
- Well tolerated by fish
- Kills all parasitic stages of sea lice
- Effective at all sea temperatures
- Sustained efficacy for up to 10 weeks
- Minimal environmental effect
Emamectin is a member of the chemical class of avermectins, macrocyclic lactones, produced by
fermentation of the soil actinomycete, Streptomyces avermitilis. Chemical modification of this
fermentation product has yielded hundreds of analogues3 including ivermectin, abamectin, moxidectin and doramectin which are widely used for control of animal and human parasites as well as insects and mites on crops.4
The first member of the avermectin family, ivermectin, to be developed as an antiparasitic agent for livestock possessed both contact and systemic activity against immature and adult ectoparasites. Thus it was logical that it would be tested in salmon for the control of sea lice. Ivermectin administered in feed proved efficacious against both chalimus and motile stages.5-8 Ivermectin used for sea lice control has a duration of efficacy of
approximately one month and can be toxic to fish, thus requiring a pattern of use that allows
only twice weekly applications.9-11 In many of the salmon-producing areas, ivermectin has been employed as a lousicide for salmon without regulatory approval for this application2.
This has resulted in veterinarians recommending an extended withdrawal period because a maximum residue limit (MRL) for ivermectin in salmon tissues has been neither
requested nor approved. When the manufacturer made a considered decision not to support the development of ivermectin for aquaculture, it was in the belief that their research effort on second generation avermectins would yield a compound with significant advantages over ivermectin for use in aquaculture. Emamectin benzoate, the active principle of SLICE, is such a compound.
Other registered products available for control of sea lice either require the use of bath treatments or they do not effectively control all parasitic stages of sea lice or they provide little sustained efficacy.
SLICE was developed by the Research Division of Schering-Plough Animal Health specifically
to provide producers with a product that combines highly effective control of all parasitic stages of sea lice with safety for fish, ease of administration and sustained duration of efficacy for up to 10 weeks.
Chemistry:
The following data describes the active ingredient: Emamectin benzoate is the active ingredient in SLICE. Emamectin is a 16-member macrocyclic lactone, 4deoxy4epimethylaminoavermectin B1, which is a mixture of two active homologous compounds:
- 4deoxy4epimethylaminoavermectin B1a (minimum 90%)
- 4deoxy4epimethylaminoavermectin B1b (maximum 10%)
The mixture of these two homologues is termed emamectin. Emamectin is obtained synthetically
from the natural avermectins B1a and B1b (collectively abamectin or avermectin B1), which differ
from emamectin by the presence of a hydroxyl group at the 4 position rather than the
4epimethylamino group.
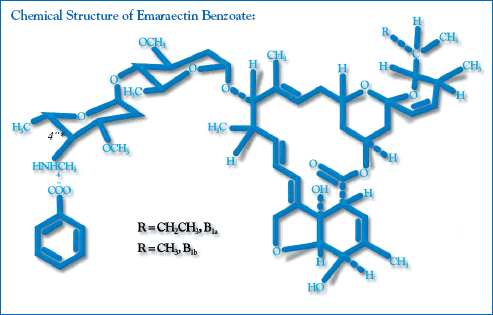
Scientific Name:
(4R)-5-O-demethyl-4deoxy-4(methylamino)avermectin A1a and (4R)-5-O-demethyl-25- de (1-methylpropyl)-4-deoxy-4-(methylamino)-25-(1-methylethyl) avermectin A1a (9:1)
Generic Name:
Emamectin benzoate
Molecular Formula:
B1a component C49H75NO13C7H6O2
B1b component C48H73NO13C7H6O2
Molecular Weight:
B1a component: 1008.26 gm/mole
B1b component: 994.24 gm/mole
Dosage Form:
Premix
| Formula for SLICE (Emamectin Benzoate 0.2% Aquaculture Premix): | ||
| Components | Percent (%w/w) | |
| Emamectin Benzoate | Inert Ingredients | |
| 0.2 | 99.8 | |
- Supplied in 2.5kg sachets containing 5g of emamectin benzoate (0.2% w/w)
- Emamectin Benzoate 0.2% Aquaculture Premix has a shelf life of 24 months
- The medicated feed can be manufactured by either a dry-coating method or a wet-coating method.
- Rate of incorporation into non-medicated feeds for a 0.5% feeding rate:
- One [2.5kg] sachet of SLICE Premix + 497.5 kg feed = 500 kg medicated feed
- Two [2.5kg] sachets of SLICE Premix + 995 kg feed = 1,000 kg medicated feed
Dose Rate
Recommended dose rate is 50 g/kg of fish biomass per day for 7 consecutive days
Suggested feeding rate for medicated feed = 0.5% of total weight of fish per pen. If the feeding rate differs from 0.5% biomass/day, then the concentration of SLICE in feed must be adjusted proportionately.
For 1,000 kg of fish administer: 5.0 kg of medicated feed per day / 35.0 kg per week
Mechanism of Action:
 |
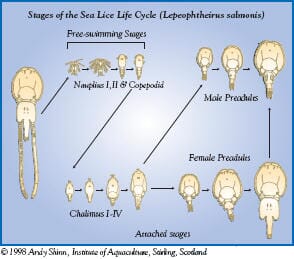
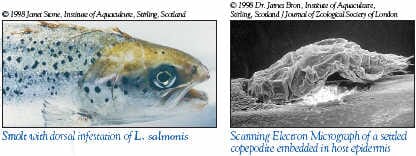
| L. salmonis chalimus stage |
The chalimus stages attach to the skin by a frontal filament and are not motile so it is most likely that they are exposed to SLICE during feeding on mucus, skin and plasma. The pre-adult and adult stages are motile so they move in the mucus covering the skin in addition to ingesting mucus, skin, plasma, and subcutaneous tissue. Therefore, the motile stages of sea lice most likely are exposed to SLICE by contact with mucus and by feeding on various fish tissues and fluids.
The precise mechanism by which SLICE (emamectin benzoate) kills sea lice has not been fully elucidated, but through extensive research the general mode of action for the avermectin class of compounds has been determined. The mechanism of avermectin killing is disruption of chloride ion movement in nerves and thus neurotransmission through competitive binding to glutamate-gated chloride channels of invertebrate nerves.16-19 This mode of action differs from that of organophosphates that inhibit neurotransmission in sea lice by disruption of cholinesterase activity and of insect growth regulators that inhibit chitin synthesis to disrupt cuticle formation. This unique mode of action should reduce the potential for cross-resistance with other approved products used for sea lice control.
Pharmacokinetics
Absorption, distribution, metabolism and excretion studies using radiolabeled emamectin benzoate were conducted in rats, bluegill sunfish, salmon, chickens, goats and Rhesus monkeys.20-25 Conclusions of these studies were consistent for all the species in that emamectin benzoate was; a) well absorbed, b) rapidly excreted, nearly all in the feces, and c) the major residue with the minor metabolite (desmethylamino emamectin). Results of repeat dosing studies in rats and bluegill sun-fish further confirmed that emamectin benzoate is not a bioaccumulative compound.
Studies in Salmon
Atlantic salmon, Salmo salar, was the representative salmonid species used for two studies to determine the fate of emamectin benzoate; a whole-body autoradiography study and a radiolabeled residue depletion study conducted at both 5C and 10C. Results from the whole body autoradiography study indicated that emamectin benzoate was a) absorbed from the gastrointestinal tract and transferred to other tissues, b) widely distributed in salmon tissues including the skin, c) present in higher concentrations in skin than in muscle, and d) excreted slowly because of enterohepatic circulation.
In the radiolabeled residue depletion study, salmon received medicated feed with radiolabeled
emamectin benzoate at a dose rate of 50 g/kg for seven consecutive days. Results showed that: a)
maximum radioactivity concentrations in muscle and skin occurred within 72 hours after administration and declined thereafter, b) tissue radioactivity declined faster at 10C than at 5C indicating that emamectin benzoate is cleared from tissues faster at higher temperatures,
c) radioactivity concentrations were lower in muscle than in skin and depleted somewhat faster
from muscle than from skin, d) emamectin benzoate was present at low concentrations in the mucus covering the skin for an extended period of time, e) at either water temperature, mean radioactivity concentrations in the edible tissues (muscle/skin) never exceeded 80 ppb (microgram equivalents of emamectin per kg) at all time intervals tested from 3 hours to 90 days after fish received the final dose of medicated feed.25
Toxicology
A complete toxicological evaluation has been conducted with emamectin benzoate. These
include extensive unpublished and published studies in mice, rats, birds26, rabbits, dogs and monkeys.23 Results from one-year and two-year sub-chronic and chronic studies demonstrated that emamectin benzoate was not carcinogenic and as a result a No Observed Effect Level (NOEL) of 0.25 mg/kg was established. Results from another series of studies conducted to evaluate potential mutagenic and teratogenic effects showed that emamectin benzoate was not mutagenic and caused no teratogenic effects. Studies on reproductive and developmental toxicity resulted in a NOEL of 0.6 mg/kg.
Maximum Residue Limit (MRL) - Europe: An Annex I maximum residue limit of 100 g/kg has
been recommended for salmonids in Europe.
Withdrawal Period:
Withdrawal times for SLICE have not been set but may be based on the recommended European MRL of 100 g/kg and the results of both radiolabeled and non-radiolabeled residue studies in salmon. It is expected that when the withdrawal times are established they will not affect commercial use of the product in strategic parasite control programs.
Salmon and Trout Safety Studies:
Target animal safety and tolerance studies were performed with SLICE on two salmonid species: Atlantic Salmon (Salmo salar) and Rainbow Trout (Oncorhynchus mykiss). A summary of the results for these two studies is presented below.
Salmon Safety Study
This study measured the tolerance of Atlantic salmon (Salmo salar) to an orally administered,
SLICE-medicated feed. Each treatment group contained 50 Atlantic salmon with a mean
bodyweight of 382g. The treatment groups were fed a SLICE-medicated diet at nominal dose rates
of 0, 100, 250 and 500g/kg/day respectively for 7 consecutive days.
All fish were observed daily for 13 days, with data recorded for mortality, behavior and overall
appearance. Following completion of the study on Day 13, all salmon in the trial were killed and
examined by gross necropsy and histopathology.
| Results of Atlantic Salmon Tolerance Study | ||
| Daily Rates* in g/kg/day [for 7 consecutive days] |
Multiple of target dose [based on 50g/kg/day] |
Observed Results |
| 0 | 0x | No Adverse Reaction |
| 70 | 1.4x | No Adverse Reaction |
| 173 | 3.5x | No Adverse Reaction |
| 356 | 7.1x | Progressive signs of toxicitylethargy, dark coloration, inappetence, loss of coordination |
| * actual dose rates were calculated based on the measured feed consumption and analysis of feed for emamectin benzoate concentration. | ||
Distinct signs of toxicity were observed only at the highest dose rate. The signs observed were dark coloration, inappetence, lethargy and in about 10% of fish a loss of coordination. No pathognomonic signs of emamectin benzoate toxicity were identified during gross necropsy or histopathological examination. No treatment-related mortality was observed.
Results from this salmon safety study showed that SLICE-medicated feed, when administered at actual dose rates (based on feed analysis) of up to 3.5x the recommended label dose rate of 50 g/kg/day, is safe for salmon.
Trout Safety Study
Trials involving 128 Rainbow trout (Oncorhynchus mykiss) with a mean weight of 295g were conducted to determine their dietary tolerance to emamectin benzoate. Sixteen of these seawateradapted trout were placed in each of 8 experimental tanks, with two tanks selected for each of the following SLICE-medicated feeding regimes: (nominal dose rates) 0, 100, 250 and 500 g/kg/day for 7 consecutive days. The dose rates of this medicated daily diet represented multiples of 2x, 5x and 10x respectively, of the recommended therapeutic dose rate of 50 g/kg/day.
Daily observations were made for appearance, behavior and mortality for 13 consecutive days. All
trout were then killed and examined by gross necropsy. In addition, five apparently healthy trout
were also examined histopathologically. Results of the gross necropsy examinations were negative.
The results of this study showed that the SLICE-medicated diet was safe for trout even when
fed at dosage rates of up to 4.4x the prescribed label dose rate.
| Results of Rainbow Trout Tolerance Study | ||
| Daily Rates* in g/kg/day [for 7 consecutive days] |
Multiple of target dose [based on 50g/kg/day] |
Observed Results |
| 0 | 0x | No Adverse Reaction |
| 88 | 1.8x | No Adverse Reaction |
| 218 | 4.4x | No Adverse Reaction |
| 413 | 8.3x | Progressive signs of toxicitylethargy, dark coloration, inappetence, loss of coordination. |
| * actual dose rates were calculated based on the measured feed consumption and analysis of feed for emamectin benzoate concentration. | ||
Distinct signs of toxicity were observed only at the highest dose. Signs of toxicity included increased melaninization (dark coloration), lethargy and inappetence. No pathognomonic signs of emamectin benzoate toxicity were identified during gross necropsy or histopathological examination. No treatment-related mortality was observed.
Environment
With the possible exception of hydrogen peroxide baths administered for the control of sea lice, all chemotherapeutants currently available have the potential of causing environmental damage,
depending on the exposure level.2 Environmental exposure is dependent upon a variety of factors
including: the amount of active ingredient(s) used for treatment, the frequency of use, the biological activity of the active ingredient, the biological activity of any metabolites or degradation products, the degree of deposition and the sensitivity of the surrounding biota.
SLICE is administered in pelleted feed that is largely consumed. As a result, deposition into the
surrounding environment may occur by two routes:
- Emamectin benzoate in uneaten feed that falls to the sea floor
- Emamectin benzoate and the desmethylamino metabolite excreted in feces of treated fish

Extensive data relative to the potential environmental impact of emamectin benzoate use in terrestrial environments27-32 have been generated during the development of emamectin benzoate for control of insects on high value food crops intended for human consumption.33-37
From these studies and others undertaken by Schering-Plough Animal Health a comprehensive
data set has been developed on the toxicity of emamectin benzoate to invertebrates, fish, birds and mammals. This has enabled predictive risk assessments to be undertaken in which 100 fold assessment (safety) factors have been applied to the toxicity data for the most sensitive of the species appropriate for the environments considered. Comparison of the predicted no effect concentrations (PNEC) with the predicted environmental concentrations (PEC) in water during, and
following, treatment indicates that the therapeutic use of emamectin should not affect invertebrates or vertebrates in the water.38
While the administration of emamectin benzoate in feed to fish reduces the overall inputs to the
environment it increases the potential for deposition in sediments. Conservative models have
been applied to commercial use scenarios allowing for uneaten feed and excretion of parent
compound and the primary metabolite from fish. The resultant PECs have indicated that any
potential for adverse effects on sensitive sediment-dwelling biota would be limited to the immediate vicinity of the treated farm.
To determine the extent of any impact, an extensive monitoring program was undertaken in a
Scottish sea loch over 12 months following the use of SLICE to treat salmon under commercial
conditions. Emamectin was detected in settling particulate material downstream of the cages but
the levels detected in sediments were lower than predicted by the models, indicating that it was
not accumulating, but being dispersed at low concentrations. Levels in sediments exceeding the
PNEC for sediment dwelling organisms were detected only within 10 meters of the cages and
lower levels were found at 12 months versus the four month time point. The PNEC for sediment
dwelling organisms is more than four times the limit of detection in sediments thus ensuring that
any potential risk can be identified by the validated methods.
Monitoring of the sediment dwelling populations in the vicinity of the treated farm did not detect any changes in communities that could be attributed to treatment. Similarly, no interference was found with the seasonal rhythms in surface fauna or macrobenthic infauna. Whilst emamectin benzoate was detected in mussels immediately following treatment it was never found at quantifiable levels and was rapidly depurated such that within 1 month it could only be detected 10 meters downstream of the cages. Emamectin benzoate was detected in fish and invertebrates in
the vicinity of the treated farm but never at levels greater than 4% of the proposed MRL
(maximum residue limit) for salmon muscle/skin. The concentrations declined such that no
quantifiable levels could be detected by 4 months after treatment.
Emamectin benzoate is absorbed in salmon and extensively metabolized with approximately 50%
of the ingested dose being metabolized before excretion. Metabolism is rapid with the metabolite
being detected in settling material and sediment following treatment. The reductions in
emamectin benzoate levels in sediments were accompanied by the appearance of the degradation
metabolite, the desmethyl amino metabolite, at non-quantifiable levels in samples taken in the
field at 12 months. The toxicity of the metabolites of avermectins have been found to be less than those of the parent compounds.
Results from field efficacy studies indicate that SLICE can provide effective sea louse control for up to 10 weeks following a single treatment. Compared to other sea louse control agents that may have to be applied as often as twice/month, the use of SLICE should result in fewer applications for effective sea louse control. The duration of efficacy together with exceptional control of all parasitic stages should reduce future sea louse populations and minimize the quantities of compound delivered into the marine environment.
Efficacy: Results of Clinical Studies - Overview
The efficacy of SLICE (emamectin benzoate) as a treatment for sea lice infestations on Atlantic
salmon was evaluated through an extensive series of clinical studies and field trials. Initially,
emamectin benzoate was used in an unformulated state and incorporated in an edible oil for application as a coating on pelleted feed. Dose rate determination and dose rate confirmation studies were conducted at several locations throughout Scotland.
Small field trials were conducted in Scotland, Norway, Canada, and Chile. In these field trials, fish were fed a commercial feed treated with SLICE, 0.2% emamectin benzoate aquaculture premix. A
daily diet of medicated feed was administered at the recommended dose rate of 50 g/kg
biomass/day for 7 consecutive days.
Commercial field trials were conducted in Canada, Chile, Scotland, and Norway. In Norway the
efficacy of SLICE was compared to that of another in feed treatment, teflubenzuron, a chitin synthesis inhibitor known commercially as Ektobann. Results from four study sites in Norway
showed SLICE medicated feed provided better sustained efficacy against sea lice when compared
to teflubenzuron-treated diets.
Characteristics of SLICE:
High level efficacy: SLICE rapidly killed all parasitic stages (motile and non-motile) of sea lice.
Duration of efficacy: SLICE killed all stages of sea lice including gravid adult females for up to 10 weeks.
Efficacy unaffected by Temperature: SLICE was effective under a wide range of environmental conditions, i.e. water temperatures of 5-15C and salinity from 23-35 ppt.
Well Tolerated: SLICE was well tolerated with no mortality or significant reduction in feeding associated with treatment.
The clinical evaluation of efficacy for the control of sea lice using emamectin was conducted as follows:
- Dose Titration and Dose Confirmation in Salmon
- Efficacy Field Trials (Scotland)
- Dose Confirmation in Trout
- Commercial Field Trials (Scotland, Norway, Chile, and Canada)
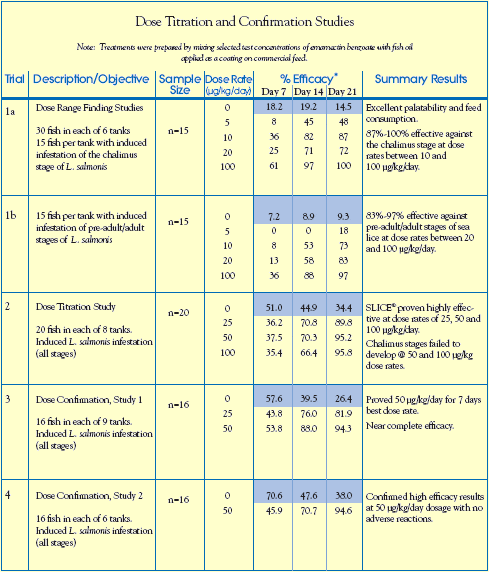
Dose Titration and dose confirmation in salmon:
Four trials were conducted in Scotland over a two-year period to determine and confirm the optimum dose rate of emamectin benzoate. Atlantic salmon (Salmo salar) ranging from 150 - 400
grams body weight were given a pelleted feed coated with emamectin benzoate in fish oil.
During each of these trials, treatment groups were fed the emamectin benzoate-medicated diet at
various dose rates for 7 consecutive days from Day 0 to Day 6. Control groups were fed the same
unmedicated commercial feed at the same rate. Efficacy was assessed by counting the number of
sea lice on all fish at Day 7, Day 14 and Day 21.
A total of 580 Atlantic salmon were utilized in the dose titration and dose confirmation studies,
with 414 fish in treatment groups that received medicated feed and 166 fish in the control groups
fed an unmedicated diet.
Results and Significant Findings
Results of trials 1a and 1b (see table, opposite) indicated that a emamectin benzoate medicated
diet given to fish for 7 consecutive days (at dose rates between 20 and 100 g/kg/day) resulted in effective control of both non-motile (chalimus) and motile (pre-adult and adult) stages of sea lice.
Trial 2, designed to determine the optimal dose rate of emamectin benzoate, indicated that a medicated diet fed at a dose rate of 25 to 50 g/kg/day for 7 days, provided control of all parasitic stages of the sea louse, Lepeophtheirus salmonis. This was further validated in subsequent Dose Confirmation Trials (3 and 4), which resulted in the selection of 50 g/kg/day as the dose rate for commercial product development.
Fish consumption of the emamectin benzoate medicated feed equaled or exceeded feed intake
rates of unmedicated control groups, thus indicating similar palatability. Emamectin benzoate
treatments were physiologically well tolerated by all groups, with no adverse reactions or mortality observed at or above the selected dose regime.
Shaded area indicates the mean number of sea lice counted per untreated fish.
% Efficacy was calculated using Abbotts formula based on the geometric mean number of sea lice per fish.
Control fish treated with hydrogen-peroxide.
Dose confirmation in Trout
A sea-pen efficacy study conducted with Rainbow trout (Oncorhynchus mykiss) in Chile further validated the efficacy of emamectin benzoate against natural infestations of two other Caligus sp.: C. flexispina and C. teres.
Study Guidelines
Housing: (6) 7m x 7m x 7m Trial Cages (500 Trout per Cage)
Treatment Design: Allocated into 3 x 2 replicates (each replicate consisted of a SLICE-treated pen and an untreated pen)
Sea Water Temperature: 11.0C - 12.5C
Salinity Range: 30 - 32 ppt
Infestation: Naturally infested with sea lice, Caligus flexispina and C. teres
Study Design: Treatment Group: fed SLICE-medicated diet at a target dose rate of 50g/kg/day for 7 consecutive days, from Day 0 to Day 6
Control Group: received unmedicated feed throughout the trial.
Data-Gathering Criteria: Trout were randomly selected from each cage on Study Days - 3, 7, 14, 21, 28, 35, 42. Sea lice were counted on 15 fish from each cage.
Results: SLICE efficacy against sea lice on trout was equivalent to that observed against
sea lice on salmon. The efficacy of SLICE was approximately 90% by 8 days after
treatment and remained >93% for at least 36 days after treatment.

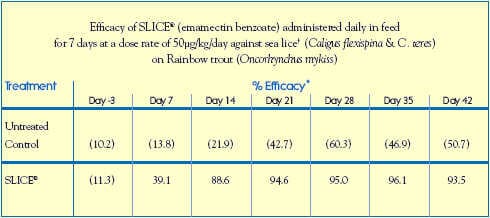
( ) numbers in parentheses are the mean numbers of sea lice / fish.
Trout were naturally infested with chalimus, pre-adult and adult stages.
* percent efficacy was calculated by Abbotts formula based on the arithmetic mean number of sea lice/fish.

Commercial Field Trials
The performance of SLICE for sea lice control was confirmed under commercial conditions in each of the major salmon producing countries; Scotland, Norway, Chile and Canada. A total of approximately 870,000 Atlantic salmon were treated in these trials.
Scotland
Parameters and results of this study are detailed below.
Housing: (16) 15m x 15m x 9m Commercial Pens with 14,163 - 15,961 Atlantic Salmon (Salmo salar) per pen.
Treatment Design: 12 treated pens (total of 184,908 fish) and 4 untreated pens
(total of 62,435 fish).
Sea Water Temperature Range: 9.8 oC - 14.0oC
Salinity Range: 13.0 - 31.5ppt (at the surface)
Infestation: All fish naturally infested with L. salmonis, and C. elongatus with
continual reinfestation pressure during the entire study.
Study Design:
Treatment Group: Fed SLICE-medicated feed at 50g/kg/day for 7 consecutive days,
Day 0 to Day 6, then unmedicated feed throughout the remaining trial period.
[Represents feeding at a rate of 1.0% biomass/day-0.5% SLICE-medicated diet,
followed by 0.5% unmedicated diet during the treatment period].
Control Group: Fed unmedicated feed at rate of 1.0% biomass/day.
Feed Preparation: SLICE-medicated feed was prepared at a commercial feed mill by coating
SLICE premix onto pelleted feed, with the addition of a final coating of fish oil.
Data-Gathering Criteria: 10 fish were randomly selected from 4 treated pens and 4 untreated pens for counting of sea lice on Study Days: -1, 13, 27 and 77. In addition, sea lice were counted on 5 fish from 1 untreated pen and 1 treated pen on Study Days: 34, 42, 49, 54, 64 and 72.
Results:
Feeding a SLICE-medicated diet proved to be over 90% effective in the control of sea lice for 58 days after treatment.
Reduced Skin Damage: 70 Days after treatment, approximately 50% of untreated fish had dermal lesions from sea lice infestation, while less than 10% of SLICE-medicated salmon showed sea lice damage.
Reduced Sea Lice Reproductive Potential By 80%: For up to 70 days after treatment, the percentage of gravid (egg-bearing) sea lice females on SLICE-medicated salmon was reduced by 80%. This reduction in gravid females may have dramatic impact on future sea lice populations within the SLICE treatment area.
Well Tolerated: Fish fed a SLICE-medicated diet exhibited no adverse health effects or mortality related to this treatment.
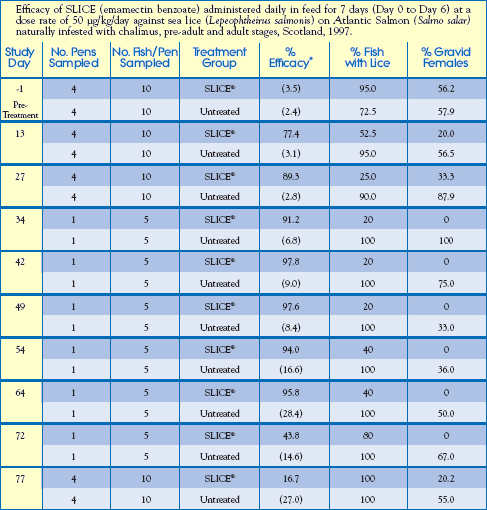

( ) numbers in parenthesis are the mean number of sea lice/fish.
* percent efficacy was calculated by Abbotts formula based on the geometric mean number of sea lice/fish.
Commercial Field Trial: Norway
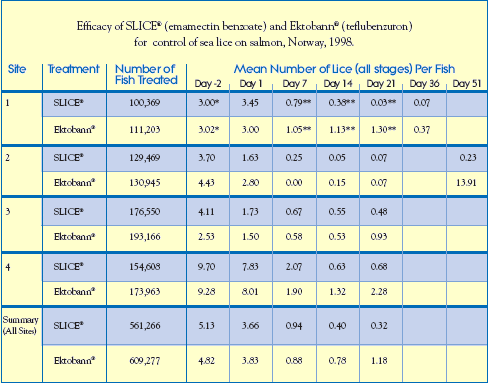
SLICE (Emamectin) vs. Ektobann (Teflubenzuron, Skretting AS) In these trials conducted at four different sites in Western Norway, a total of 1,170,543 Atlantic salmon were treated with either SLICE (emamectin benzoate) or Ektobann (teflubenzuron, Skretting AS) at their recommended therapeutic dose rates.
In these trials conducted at four different sites in Western Norway, a total of 1,170,543 Atlantic salmon were treated with either SLICE (emamectin benzoate) or Ektobann (teflubenzuron, Skretting AS) at their recommended therapeutic dose rates.
The SLICE treatment groups of 561,266 salmon received a target dose rate of 50g/kg bodyweight/day for 7 consecutive days. Salmon in the Ektobann treatment groups numbering 609,277 were administered a target dose rate of 10mg/kg bodyweight/day, also for 7 consecutive days.
Salmon in these Norwegian trials ranged in size from 92g to 347g. All were held under commercial rearing conditions and fed a SLICE-medicated diet or an Ektobann-medicated diet at the rate of 0.5% biomass/day throughout the treatment period.
The study guidelines outlined below were consistent for each of the 4 trial sites.
Housing: (6) Commercial Rearing Pens
Treatment Design: Allocated randomly into 3 x 2 replicates (each replicate consisted of a SLICEtreated pen and an Ektobann-treated pen)
SeaWater Temperature: 12.8C - 15.8C
Infestation: Naturally infested with primarily L. salmonis and secondarily C. elongatus.
Data-Gathering Criteria: Salmon (20) were randomly selected from each cage and killed on Study Days -2, 1, 7, 14, 21, 36, 51* (Study Day 0 was the first day of treatment).
Sea lice in all stages (chalimus,pre-adult, adult) were counted.
* indicates sea lice were counted 1 day earlier than shown in the column heading.
** indicates sea lice were counted 1 day later than shown in the column heading.
Results
In all four of these Norwegian studies, there was an excellent response to SLICE therapy within 7 days of beginning treatment. After 21 days, the mean number of sea lice on fish that received the SLICE medicated diet was significantly lower than those treated with Ektobann. This efficacy differential continued following observations on Day 36 and again on Day 51. At Site 2, the mean number of sea lice 51 days after treatment on salmon treated with SLICE was 0.23 lice/fish, while fish administered Ektobann presented increased re-infestation levels with an average of 13.9 sea lice per fish.
Separate analysis (data not shown) of the efficacy against individual stages of sea lice showed that no chalimus were found on SLICE-treated fish by Day 36 (Site 1). At Site 2, chalimus numbers on treated fish increased by Day 51 to 0.23 chalimus/SLICE-treated fish and 10.5 chalimus/Ektobann-treated fish. In addition, no preadults or adults were found on SLICE-treated fish on Day 51 (Site 2).
Commercial Field Trial: Canada
An efficacy study with Atlantic salmon was conducted at 2 commercial sea farm sites in eastern Canada. Four (4) cages at each site were selected for the study, i.e. 2 cages received SLICE-medicated feed and 2 cages were untreated. A total of 76,210 fish were treated with SLICE and 75,141 fish started the study as untreated controls. Fish weighed approximately 470 gm at the start of the study and received a target dose rate of 50 g/kg biomass/day for 7 consecutive days, Day 0 to Day 6. Feeding rates for the sites were 2.7% and 3.1% biomass/day, respectively.
Parameters and results of this study are detailed below.
Study Guidelines:
Housing: (8) Commercial rearing pens.
Treatment Design: 2 replicates (1 treated pen & 1 untreated pen / replicate) per site.
Infestation: Fish were naturally infested with sea lice, primarily Lepeophtheirus salmonis and
secondarily with Caligus elongatus.
Data Collection Criteria: Salmon (10) were randomly selected from each cage by hand net at each sea farm site on Study Days, -5/-6, 7/8, 14/16, 22, 28/29, and 43/44. Additionally,
at one site, 10 fish were sampled on Study Days 57 and 73 and 5 fish were
sampled on Study Days 92 and 115. Fish were anesthetized and the number of
sea lice (chalimus, pre-adult/adult, gravid females) were counted on each fish.
Results: Data were analyzed for each sea lice stage; non-motile, motile and gravid female.
The percent efficacy based on the total number of sea lice/fish is shown in the
table opposite. The untreated control pens had to be treated with Salmosan
(azamethiphos, Novartis) on three occasions during the study, Site 1 on Study
Days 9, 26 and 34 and Site 2 on Study Days 10, 33 and 58. As a result of the
control group treatments, the calculated efficacy was lower than would otherwise
have been observed. The duration of the clinical effect of SLICE on sea
lice populations was confirmed statistically through 44 days and despite considerable
reinfestation pressure nearly complete sea louse control was observed
through 67 days after treatment (Study Day 73). Efficacy declined from 79% on
Study Day 92 to 63% on Study Day 115 when deteriorating weather conditions
prevented further monitoring of louse populations. Pre-adult, adult and gravid
female sea lice were virtually eliminated on fish treated with SLICE. Salmon
fed readily on SLICE-medicated feed and no adverse events were observed
during the study.
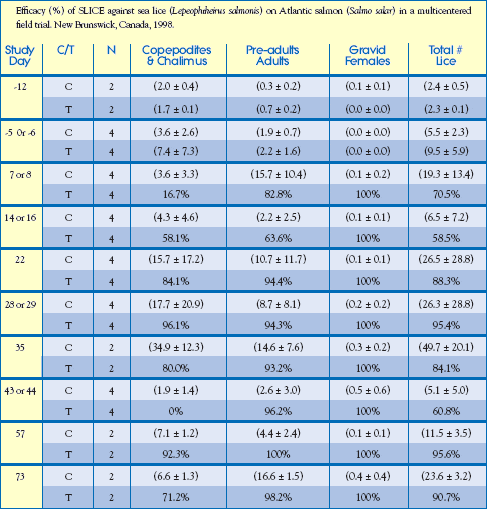
Treatment (T) or Control (C)
( ) indicate the mean number of sea lice / per fish standard deviation
Note: At one site, the efficacy was 79.2% on Study Day 92 and 62.9% on Study Day 115.
Commercial Field Trial: Chile
An efficacy study with Atlantic salmon was conducted at a commercial sea farm site in the vicinity of Puerto Montt, Chile. Six (6) cages were selected for the study, i.e. 3 cages received SLICE-medicated feed and 3 cages were untreated. Fish received a target dose rate of 50 g/kg biomass/day for 7 consecutive days, Day 0 to Day 6.
Parameters and results of this study are detailed below.
Study Guidelines:
Housing: 6 Commercial rearing pens.
Treatment Design: 3 replicates (1 treated pen & 1 untreated pen / replicate).
Infestation: Fish were naturally infested with sea lice, primarily Caligus flexispina.
Data Collection Criteria: Salmon (10) were randomly selected from each cage by hand net on Study Days,
-1, 26, 46 and 102. Fish were anesthetized and the number of sea lice (chalimus,
pre-adult/adult, gravid females) were counted on each fish.
Results: Data were analyzed for each stage; non-motile, motile and gravid female.
The percent efficacy based on the total number of sea lice/fish is shown in the
table opposite. The efficacy of SLICE exceeded 90% against all parasitic stages
of Caligus. Infestations were not monitored between Study Days 46 and 102
because of an algal bloom. Efficacy against non-motile stages and pre-adults
declined to zero during this period. However, efficacy against gravid females
exceeded 80% 95 days after treatment was concluded. The SLICE-induced
suppression of maturing reproductive females would be expected to cause eventual
reductions in reinfestation pressure. Hence, fewer sea lice treatments would
be required per growing season. Salmon fed readily on SLICE-medicated feed
and no adverse events were observed during the study.
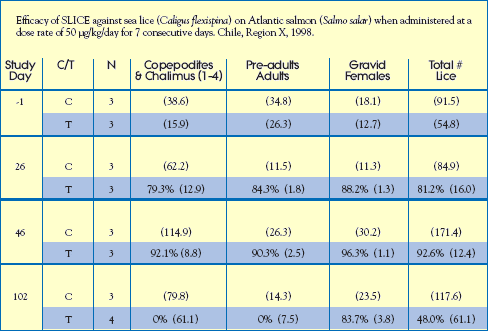
Treatment (T) or Control (C)
( ) indicate the mean number of sea lice / per fish standard deviation.
Conclusion
The high order efficacy of SLICE-medicated feed demonstrated against all stages of sea lice,
for up to 10 weeks post treatment, should lead to improved sea lice control as well as
fewer overall treatments. And, since a SLICE-treated diet can eliminate pre-adult and adult
sea lice, administering SLICE treatments early in the year could significantly reduce or delay
the development of economically significant parasite populations, where wild fish are not a
major source of reinfestation.
References
1. MacKinnon, B.M. 1997. Sea Lice: a review. World Aquaculture. 28(3): 5-10.
2. Roth, M., R. Richards & C. Sommerville. 1993. Current practices in the chemotherapeutic
control of sea lice infestations in aquaculture a review. Journal of Fish Diseases.
16(1):1-26.
3. Fisher, M.H. & H. Mrozik. 1984. The avermectin family of macrolide-like antibiotics. In:
Macrolide Antibiotics: Chemistry, Biology and Practice. pp. 553-606. Academic Press.
New York.
4. Fisher, M.H. 1997. Structure-activity relationships of the avermectins and milbemycins.
Phytochemicals for Pest Control. pp 220-238, ACS Symposium Series 658, American
Chemical Society, Washington, DC.
5. Palmer, R., H. Rodger, E. Drinan, C. Dwyer & P.R. Smith. 1987. Preliminary trials on
the efficacy of ivermectin against parasitic copepods of Atlantic salmon. Bulletin of the
European Association of Fish Pathologists. 7:47-54.
6. Johnson, S.C. & L. Margolis. 1993. Efficacy of ivermectin for control of the salmon louse
Lepeophtheirus salmonis on Atlantic salmon. Diseases of Aquatic Organisms. 17:101-105.
7. Smith, P.R., M. Moloney, A. McElligott, S. Clarke, R. Palmer, J. OKelly & F. OBrien.
1993. The efficiency of oral ivermectin in the control of sea lice infestations of farmed
Atlantic salmon pp. 296-307. IN: Pathogens of Wild and Farmed Fish, ed. G.A. Boxshall
& D. Defaye. Ellis-Horwood, London.
8. Sievers, G., M.C. Ramirez & F. Muller. 1996. Efectividad de la ivermectina sobre el
ectoparasito Caligus sp. en truchas cultivadas (Oncorhynchus mykiss) en el sur de Chile.
Archivos de Medico Veterinarios. 28(1):93-99.
9. Palmer, R., R. Coyne, S. Davey & P. Smith. 1997. Case notes on adverse reactions associated
with ivermectin therapy of Atlantic salmon. Bulletin of the European Association
of Fish Pathologists 17(2): 62-67.
10. Kilmartin, J., D. Cazabon & P. Smith. 1997. Investigations of the toxicity of ivermectin
for salmonids. Bulletin of the European Association of Fish Pathologists. 17(2):58-61.
11. Johnson, S.C., M.L. Kent, D. J. Whitaker, & L. Margolis. 1993. Toxicity and pathological
effects of orally administered ivermectin in Atlantic, chinook, and coho salmon and
steelhead trout. Diseases of Aquatic Organisms. 17:107-112.
12. Kabata, Z. 1974. Mouth and mode of feeding of Caligidae (Copepoda), parasites of fishes,
as determined by light and scanning electron microscopy. Journal of the Fisheries
Research Board of Canada. 31: 1583-1588.
13. MacKinnon, B. 1993. Host response of Atlantic salmon (Salmo salar) to infection by sea
lice (Caligus elongatus). Canadian Journal of Fisheries and Aquatic Sciences. 50:789-792.
14. Brandal, P.O., E. Egidius & I. Romslo. 1976. Host blood: a major food component for the
parasitic copepod Lepeophtheirus salmonis Kroyer, 1838 (Crustacea: Caligidae).
Norwegian Journal of Zoology. 24: 341-343.
15. Jones, M.W., C. Sommerville & J. Bron. 1990. The histopathology associated with the
juvenile stages of Lepeophtheirus salmonis on the Atlantic salmon, Salmo salar L. Journal
of Fish Diseases. 13:303-310.
16. Arena, J.P., K.K. Liu, P.S. Paress, E.G. Frazier & D.F. Cully. 1995. The mechanism of
action of avermectins in Caenorhabditis elegans: correlation between activation of glutamate-
sensitive chloride current, membrane binding, and biological activity. Journal of
Parasitology. 81(2):286-294.
17. Duce, I.R., N.S. Bhandal, R.H. Scott & T.M. Norris. 1995. Effects of ivermectin on
gamma-aminobutyric acid and glutamate-gated chloride conductance in arthropod
skeletal muscle. IN: J.M. Clark, ed. Molecular action of insecticides on ion channels.
American Chemical Society. Washington, USA.
18. Vassilatis, D.K., K.O. Elliston, P.S. Paress, M. Hamelin, J.P. Arena, J.P. Schaeffer, L.H.
Van der Ploeg & D.F. Cully. 1997. Evolutionary relationship of the ligand-gated ion
channels and the avermectin-sensitive, glutamate-gated chloride channels. Journal of
Molecular Evolution. 44(5):501-508.
19. Laughton, D.L., G.G. Lunt & A.J. Wolstenholme. 1997. Reporter gene constructs
suggest that the Caenorhabditis elegans avermectin receptor beta-subunit is expressed
solely in the pharynx. Journal of Experimental Biology. 200 (Pt 10):1509-14.
20. Mushtaq, M., L.R. Syintsakos, P.A. Krieter, A. Colletti, B. Arison, L.S. Crouch &
P.G. Wislocki. 1996. Absorption, tissue distribution, excretion and metabolism of
3H- and 14C-labeled emamectin benzoate in rats. Journal of Agricultural and Food
Chemistry. 44:3342-3349.
21. Chukwudebe, A.C., N. Andrew, K. Drottar, J. Swigert & P.G. Wislocki. 1996.
Bioaccumulation potential of 4-epi-(methylamino)-4-deoxyavermectin B1a benzoate
(emamectin benzoate) in bluegill sunfish. Journal of Agricultural and Food Chemistry.
44:2894-2899.
22. Mushtaq, M., L.R.S. Allen, L.S. Crouch & P.G. Wislocki. 1997. Fate of 3H and 14Clabeled
emamectin benzoate in lactating goats. Journal of Agricultural and Food
Chemistry. 45:253-259.
23. Wrzesinski, C.L., W.P. Feeney, W.F. Feely & L.S. Crouch. 1997. Dermal penetration of
4-(epi-methylamino)-4-deoxyavermectin B1a benzoate in the Rhesus monkey. Food
Chemistry and Toxicology. 35:1085-1089.
24. Wrzesinski, C., M. Mushtaq, T. Faidley, N. Johnson, B. Arison & L.S. Crouch. 1998.
Metabolism of 3H/14C-labeled 4-Deoxy-4- epimethylaminoavermectin B1a benzoate in
chickens. Drug Metabolism and Disposition. 26(8):786-794.
25. SCH58854: Residue depletion of SCH58854 following a multiple oral (dietary)
50 g/kg dose regimen in Atlantic salmon (Salmo salar) maintained in seawater at 5oC or 10oC. Schering-Plough Animal Health, Technical Report P6745.
26. Chukwudebe, A.C, J.B. Beavers, M. Jaber & P.G. Wislocki. 1998. Toxicity of emamectin
benzoate to mallard duck and northern bobwhite quail. Environmental Toxicology and
Chemistry 17(6):1118-1123.
27. Hicks, M.R., L.D. Payne, S.V. Prabhu & T.A. Wehner. 1997. Determination of
emamectin in freshwater and seawater at picogram-per-milliliter levels by liquid
chromatography with fluorescence detection. Journal of AOAC International
80(5):1098-1103.
28. Mushtaq, M., A.C. Chukwudebe, C. Wrzesinski, L.R.S. Allen, D. Luffer-Atlas & B.H.
Arison. 1998. Photodegradation of emamectin benzoate in aqueous solutions. Journal of
Agricultural and Food Chemistry 46:1181-1191.
29. Mushtaq, M., W.F. Feely, L.R. Syintsakos & P.G. Wislocki. 1996. Immobility of
emamectin benzoate in soils. Journal of Agricultural and Food Chemistry 44(3):940-944.
30. OGrodnick, J.S., P.G. Wislocki, J.L. Reynolds, M.Wisocky & R.A. Robinson. 1998.
Aged soil column leaching of emamectin benzoate (MAB1a). Journal of Agricultural
and Food Chemistry 46(5):2044-2048.
31. Chukwudebe, A.C., W.F. Feely, T.J. Burnett, L.S. Crouch & P.G. Wislocki. 1996. Uptake
of emamectin benzoate residues from soil by rotational crops. Journal of Agricultural and
Food Chemistry 44(12):4015-4121.
32. Chukwudebe, A.C., R.H. Atkins & P.G. Wislocki. 1997. Metabolic fate of emamectin
benzoate in soil. Journal of Agricultural and Food Chemistry 45(10):4137-4146.
33. Crouch, L.S. & W.F. Feely 1995. Fate of [14C] emamectin benzoate in head lettuce.
Journal of Agricultural and Food Chemistry. 43 (12): 3075-3087.
34. Wrzesinski, C.L., B.H. Arison, J. Smith, D.L. Zink, W.J.A. VandenHeuval & L.S.
Crouch. 1996. Isolation and identification of residues of 4-(epi-methylamino)-4
deoxyavermectin B1a benzoate from the surface of cabbage. Journal of Agricultural and
Food Chemistry 44:304-312.
35. Allen, L.S., C.L. Wrzesinski, W.F. Feely, G.A. Boss & L.S. Crouch. 1997. Incorporation
of emamectin benzoate (MK-0244) residues into soluble sugars of plants. Journal of
Agricultural and Food Chemistry 45(10):4131-4136.
36. Crouch, L.S., C.L. Wrzesinski & W.F. Feely. 1997. Fate of (14C/3H) emamectin benzoate
in cabbage, 1. Extractable residues. Journal of Agricultural and Food Chemistry
45(7):2744-2757.
37. W.F. Feely & L.S. Crouch. 1997. Fate of (14C) emamectin benzoate in cabbage, 2.
Unextractable residues. Journal of Agricultural and Food Chemistry 45(7):2758-2762.
38. McHenery, J. 1998. Expert report on the potential environmental impact of emamectin
benzoate, formulated as SLICE , for salmonids. Schering-Plough Animal Health
Corporation. Unpublished Report.
Source: Schering-Plough Animal Health - March 2004



