Identity
Ostrea edulis Linnaeus, 1758 [Ostreidae]
FAO Names: En - European flat oyster, Fr - Huître plate européenne, Es - Ostra europea
View SIDP Species fact sheet
Biological features
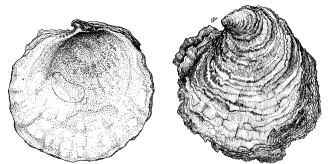
Ostrea edulis is a bivalve mollusc that has an oval or pear shaped shell with a rough, scaly surface. The irregular shell has a distinct hooked beak, patterned with delicate foliation. The two halves (valves) of the shell are different shapes subcircular to circular and inequivalve. Left shell is deeply concave and fixed to the substratum, the right being flat with rougher edges and sitting inside the left acting as a lid. Inner surfaces of both valves are smooth and usually pearly, white or bluish-grey, often with darker blue areas. Valves are held together at their narrow ends by an elastic ligament. No teeth are reported on the hinge. A large central muscle serves to close the valve against the pull of the ligament. The shell is off white, yellowish or cream in colour with light brown or bluish concentric bands on the right valve. Shell consist of a series of chalky layers which may include laminar and hollow chambers. The hard rough gray shell contains a meat that can vary in color from creamy beige to pale gray, in flavor from salty to bland, and in texture from tender to firm. O. edulis occurs from the coast of Norway to waters near Morocco, through the Mediterranean Sea, and into the Black Sea. The flat oyster can grow very large (>20 cm) and become very old (>20 years).






Profile
Historical background
The flat oyster Ostrea edulis, a native of Europe, has been part of the human diet for many centuries. The Romans built ponds to stock and sort oysters. In the 17th century, oyster spat were collected on rocks, separated from each other and deployed into ponds in salt marshes on the Atlantic coast of France. A decline in activity in salt marshes facilitated oyster culture development by expanding grow-out acreage availability. During the 18th and 19th centuries, fishing effort led to over-exploitation, failing recruitment, and destruction of European natural beds, which were also affected by extremely cold winters. Shortage in seed supply prompted the managers to develop cultural practices aimed to sustain a repletion and reseeding programme. Eventually, the 'leasing ground' system and the development of artificial spat collectors and their systematic use facilitated the development of the sector. Mostly in intertidal areas, wooden spat collectors were initially used followed by oyster shell strings and slates. Then, in 1865, the liming tile technique (roof tiles coated with a mixture of lime and fine sand), and wooden boxes to grow juveniles were developed in south-western France. Coated tiles became the main method of spat collection in France and the Netherlands: spat were removed by hand after 6-10 months, then reared in trays or re-laid in subtidal plots. On the Mediterranean coast, off-bottom culture was initiated in 1900, using oysters cemented onto steel poles. Growout facilities were developed in shallow waters (3-4 m), then oyster culture increased substantially by transferring spat from traditional spatfall areas (Brittany). Spat were cemented individually onto poles, which were hung from frameworks developed over mussel leasing grounds. This form of culture was replaced by cupped oyster (Crassostrea angulata) production in 1950.
The most obvious cultural changes during the 20th century occurred in two areas: spat collection techniques and the occurrence of disease problems in oyster populations. Spat collection techniques changed when seeding cockle (1904), then mussel (1939) shells in subtidal waters became the common practice in the Netherlands. This technique was more and more cost effective requiring far less labour. Since the 1980s, the use of tubular nets filled with mussel shell and deployed off-bottom has also proven more cost effective in southern Brittany, France. More recently, hatcheries have begun to produce cultchless flat oyster spat.
With regard to disease, a massive mortality widely struck European flat oyster populations in 1920. The population later recovered but was replaced by cupped oysters in several traditional rearing areas. Then, two diseases (Marteilia refringens and Bonamia ostrea) spread in the early 1970s and 1980s, drastically reducing the production of O. edulis in almost all European traditional rearing areas. Despite new management practices, and intensive repletion programmes, the production of O. edulis has remained low since that time.
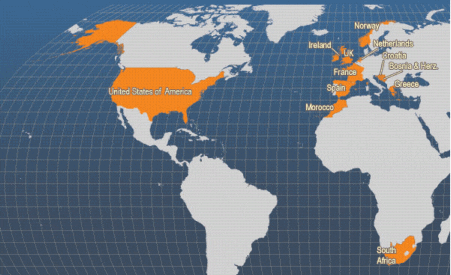
The European flat oyster is found along the western European coast from Norway to Morocco in the north-eastern Atlantic and in the whole Mediterranean Basin. Natural populations are also observed in eastern North America from Maine to Rhode Island, following intentional introductions in the 1940s and 1950s.
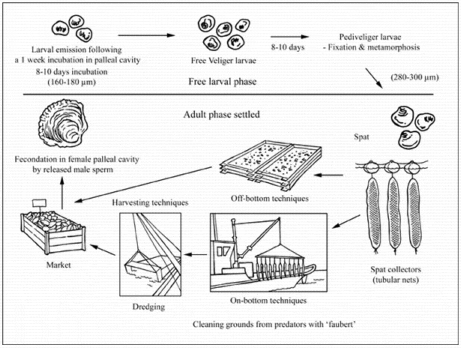
Ostrea edulis is a protandric hermaphrodite, changing sexes generally twice during a single season. Oysters function as males early in the spawning season and later change to females and vice versa. The flat oyster is usually male in the fall following its settlement. O. edulis exists as a series of physiological races, and genetic differentiation has been demonstrated along the European coastline. One of the lowest temperature races occurs in Spain where 12-13 °C is required for spawning while 25 °C is the spawning temperature in Norwegian fjords. In France, gametogenesis occurs at 10 °C and spawning between 14 and 16 °C. Female gametes are liberated into the palleal cavity where they are fertilised by externally released sperm. In contrast to the large C. gigas reproductive effort, O. edulis produces between 500 000 and 1 million eggs per spawning. Following an incubation period of 8-10 days, depending on temperature, final release into environment occurs. Then larvae (160 µm in size) spend 8 to 10 days as a pelagic stage before settlement. Appropriate larval growth and survival rates are obtained in salinities as low as 20‰, although they can survive at salinities as low as 15‰.
Production systems
Seed supply
Juveniles are obtained either through wild spat collection or by hatchery production (cultchless seed). Presently, most is wild spat from natural reproductive areas, whereas hatchery production is mostly focused in areas without natural supply. However, depleted beds and the impact of disease (Bonamia ostreae) have prompted the hatchery industry to try developing a disease resistant strain.
Broodstock
The sexual maturation and reproduction of Ostrea edulis is obtained by increasing seawater temperature and providing additional (ad libitum) food (phytoplankton). Conditioning is based on mimicking the natural reproductive cycle and environmental conditions. Compared to other traditional hatchery reared species (e.g. ) in-vitro fertilisation remains difficult for this species, resulting in an extremely low survival rate. So far, therefore, an incubation phase is still required. Synchronised maturation and spawning induction are also difficult tasks from a technical point of view. Thermal stress to induce spawning does not allow full sexual identification. Although the use of chemicals (serotonin) has facilitated reproduction control, this technique cannot be used on a routine basis. Therefore, two techniques have been developed. The first is mass spawning, based on the maturation of a whole oyster batch. This neither permits any assessment of the contribution of individual breeders to the generation nor any controlled cross-breeding. The second technique does permit controlled breeding (full siblings) by maturing only two oysters per tank and then collecting the resulting seed. All these constraints demonstrate that the entire reproductive process is still not entirely under control at the hatchery level.
Hatchery production
Hatchery rearing requires the production of suitable microalgal species as food. Usually, the phytoplankton species used are flagellates combined with diatoms to provide a well balanced diet, therefore facilitating gametogenesis and larval development. Food quantity depends on larval density. Hatchery production has remained low because no disease-resistant strain has yet been developed.
Natural spat collection
Most European flat oyster culture remains based upon the use of spat collectors to obtain wild juveniles. Spat collectors are either mussel shells, sown in June-July (18 °C), mostly in densities of 30-60 m³/ha (Netherlands) or tubular nets filled with mussel shell (each containing about 600 mussel shells) and deployed off-bottom (France). In the latter case, nets are suspended under steel frames in shallow waters (3-6 m deep). Since farmers use cooked mussel shells as spat collectors, there is no need for further work as these will naturally disaggregate. Usually, one steel frame supports around 120 tubular nets and yields between 70 to 75 kg of spat/yr. More recently, PVC dishes covered by lime have been used intertidally for this process. The main interest in using this technique is the capacity of automatic equipment to remove spat from the collectors, therefore reducing operational costs.
Nursery
Although clams have a protective shell, these will break if not handled with care during the screening and sorting processes. It is therefore necessary to collect the oysters post-setting by filtering the seawater outflow from the conditioning tank. Then the spat is cultured using traditional techniques for the micronursery and nursery stages. These include the use of micronursery trays built with nylon screens and a flow-through recirculating system with frequent water exchange (e.g. water tables, downwellers, and upwellers). Whenever the spat are removed from the water, care must be taken to ensure they do not dry out or become too warm. Once reaching 5-6 mm size (kept by a 4 mm mesh sieve), spat can be transferred using baskets to open waters for ongrowing.
Ongrowing techniques
Two main types of production techniques have been used to produce flat oysters: off-bottom and on-bottom.
Off-bottom techniques
These consist of floating trays or rafts, longlines, suspended rope, lanterns or plastic baskets hung from the rafts, intertidal trestles and oyster bags. In Spain, floating rafts like those used for mussel culture are also used for oyster culture. The technique involves manual attachment of medium size oysters (5 cm) to ropes with cement. Workers hang the ropes from rafts and periodically clean them by removing algae and mussel seed. More recently, plastic baskets have been hung from the rafts. Oysters are thinned out as they grow.
On-bottom techniques
Oyster spat are directly re-laid by boat on-bottom in subtidal grounds at a density of 50-100 kg/ha, 5 to 10 times less than for cupped oysters (Crassostrea gigas) in France. The common size at seeding, which is carried out in May-June, is 1 cm at about 1 year-old. Oyster deployment, which used to be done by manual shovelling, is now carried out either by conveyor belts or by washing out spat using seawater. Traditional management practices include the use of cotton nets to collect starfish predators, as well as teethed steel frames, being dragged in tow on the leasing grounds. Deep water culture maximises growth rate since Bonamia disease drastically reduces the survival rate of 3-4 year-old oysters. Thus, 2 year-old oysters (60-80 g) are harvested by dredging before disease-induced mortality occurs. In spite of this early harvesting, survival rates remain low, about 5 percent after a 3 year rearing cycle.
In Maine (USA), oysters are cultured in floating trays until they are in the 35-50 mm range in the late fall. Fouling is controlled biologically by the browsing periwinkle Littorina littorea. Oysters are then transferred to on-bottom leasing grounds for growth to market size. Developing effective predator control methods against starfish and crabs is the main issue for on-bottom culture.
Harvesting techniques
Since most flat oyster culture is developed in subtidal areas and in an extensive manner to avoid disease problems, oysters are usually harvested with two steel dredges about 3.5-4 m wide and 2 m deep with 3-5 cm teeth blades, operated by hydraulic or pneumatic winch.
Where intertidal culture on trestles is carried out, farmers bring the bags back to the packing houses for sorting, grading and restocking.
Handling and processing
Once oysters have reached their marketable size, farmers grade and store them temporarily in clean waters before marketing. In France, oysters are marketed according to their size (grade 0 to 5). Refrigerated trucks are commonly used to transport oysters in accordance to EU regulations.
In Spain, like other shellfish destined for the fresh market, oysters must be depurated. They are deployed into cases, into depurating tanks filled with chlorinated water. The maximum authorised weight is 30 kg/m², while the depuration time lasts 42 hours. Oysters chilled on ice are trucked to market. During sale, they are maintained at 3-10 °C.
Production costs
Although highly variable, depending on rearing site characteristics and mainly survival rate, production costs are likely to be high. This is mainly due to disease problems with this species and the very low survival rates which have prompted farmers to reduce production, but also to the length of the rearing cycle. European flat oysters are cultured extensively, and are sometimes mixed with cupped oysters (Crassostrea gigas) to reduce disease effects; this increases sorting activities. Investment is required to operate in deep waters with specific gears. Raft culture is labour intensive and requires considerable time to prepare the units as well as to clean them during the production cycle. Compared to deep water culture, which is highly mechanised using hydraulic gears and dredges, raft culture requires regular manual handling for various operations.
| DISEASE | AGENT | TYPE | SYNDROME | MEASURES |
|---|---|---|---|---|
| Herpes-like infection | Herpes virus like (OHSV-1) | Virus | No direct impact at the population level observed | No curative measures available; ensure good site selection & management practices to limit impact & compulsory oyster monitoring for transfers; zoning system |
| Shell disease | Ostracoblabe implexa | Fungus | Limited impact but black malformations on inside of shells may impede development & cause eventual mortality | No curative measures available; ensure good site selection & management practices to limit impact & compulsory oyster monitoring for transfers; zoning system |
| Bonamiasis | Bonamia ostreae | Protozoan | Most infected oysters show no obvious macroscopic symptoms; however, infections sometimes accompanied by yellow discoloration & extensive gill & mantle lesions; becomes systemic & usually lethal when oysters 2-3 years old | No curative measures available; ensure good site selection & management practices to limit impact & compulsory oyster monitoring for transfers; zoning system |
| Marteiliasis | Marteilia refringens | Protozoan | Discoloration of the digestive gland epithelia; cessation of growth; loss of tissue condition; decrease of glycogen reserves; may cause recurring mortalities | No curative measures available; ensure good site selection & management practices to limit impact & compulsory oyster monitoring for transfers; zoning system |
| Denman Island disease | Microcytos mackini | Protozoan | Green to yellow pustules or abscess-like lesions (<5 mm), resulting from haemocyte infiltration & necrosis, occur within the body wall, on the surface of labial palps or mantle, or adductor muscle; often induces brown scar adjacent to mantle surface; parasite may cause recurrent abnormal mortality rates in older oysters | No curative measures available; ensure good site selection & management practices to limit impact & compulsory oyster monitoring for transfers; zoning system |
Preventive measures
With regard to shellfish regulations, preventive measures aim to limit imports only from countries where no outbreak of disease occurs, according to the list specified by OIE International Aquatic Animal Health Code (notifiable pathogens). The three parasites mentioned in the table above are notifiable pathogens, therefore limiting any flat oyster transfer from enzootic to disease-free areas. Imports of molluscs should occur only from countries where no outbreak of disease caused by those agents has occurred for at least the two previous years and no parasite has been detected in any mollusc tested during an official health surveillance programme using the procedures described by the OIE, for at least two years. Moreover, European zoosanitary directives have enforced a zoning system to limit the expansion of these diseases. Therefore, monitoring O. edulis populations and parasites is critical to prevent and limit associated risks. Without cure, preventative measures have been adopted to limit disease effects, mostly concerning management practices, including site selection and density reduction.
Suppliers of pathology expertise
Assistance can be obtained from the following sources:
- European Shellfish Zoosanitary Reference Laboratory (OIE Reference laboratory for Marteilia and Bonamia diseases), IFREMER La Tremblade, BP 133, 17390 La Tremblade, France.
- Department of Fisheries & Oceans, Pacific Biological Station, Nanaimo, BC, Canada V9R 5K6.
- Instituto de Investigaciones Marinas Consejo Sup. de Invest. Cie., Eduardo Capelo 6, 36208 Vigo, Spain.
- CEFAS, Weymouth Laboratory, Barrack Road, The Nothe, Weymouth Dorset, DT4 8UB, UK.
- CIDC Lelystad, Institute for Animal Disease Control, PO Box 2004, 8203 AA Lelystad, The Netherlands.
Statistics
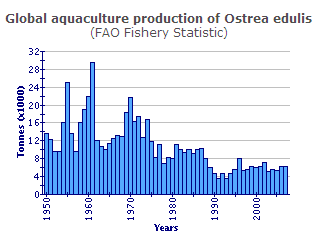
Within the past forty years production of Ostrea edulis showed a drastic decline from a peak output of nearly 30 000 tonnes in 1961, due to the impact of diseases and a consequential shift to the rearing of the Pacific cupped oyster (Crassostrea gigas). European flat oyster production has remained low throughout the decade 1993-2002; output peaked in 1996 (7 996 tonnes) but became more stable (6 000-7 000 tonnes) in 2000, 2001 and 2002.
In 2002, 67 per cent of the production was in Spain (4 565 tonnes) and 24 per cent in France (1 600 tonnes). Ireland and the UK were the only other countries that produced more than 200 tonnes in 2002.
The production of the European flat oyster constituted less than 0.2 per cent of the total global production of all farmed oyster species in 2002. The bulk of production (97.7 per cent) came from the rearing of the Pacific cupped oyster, Crassostrea gigas. However, the value of farmed O. edulis production in 2002 was USD 24.3 million; thus its culture remains an important sector in the limited areas where it is reared.
European flat oysters are traditionally consumed fresh and eaten on the half shell. Oysters are shipped to local markets or distributed to supermarkets and restaurants. As the available supply has decreased, average prices have dramatically increased. Although fluctuating widely, depending on size and local availability, prices have reached a record high (USD 13/kg) in traditional markets in France. The wholesale average price for O. edulis is commonly 3 to 5 times greater than the cheaper Pacific cupped oyster (). Therefore, the product now occupies an economic niche, and is considered as a luxury seafood item - an expensive delicacy for specialised consumers.
Status and trends
The future of European flat oyster production is directly linked to the research capacity to eventually provide a strain resistant to Bonamiasis by genetic mass selection. Presently, the economy of hatchery production is rather focussed on polyploid Crassostrea gigas production rather than on the more technically difficult European flat oyster spat production. This explains why no rebound in flat oyster production has been observed so far. For this reason, production is likely to remain at a level only to supply a niche market in the near future.
Main issues
In spite of several management practices aimed at limiting mortality and facilitating oyster growth rates, diseases have drastically affected wild and cultured flat oyster populations. Therefore, the main issue for the industry remains to develop a disease-tolerant strain to initiate a production rebound. This implies a better understanding of the pathogenic mechanisms concerned, as well as the development of sustainable genetic breeding programmes. Although research programmes have demonstrated the feasibility and effectiveness of a mass selection approach, the development of such a strain remains to be commercialised. Moreover, this approach requires a long term commitment from the industry and private hatcheries, the latter being the main stakeholders in managing the selective breeding programme.
Responsible aquaculture practices
The currently low profile of the European flat oyster population results from past errors, including the lack of preventive measures to limit the spread of diseases, and illegal transfers of Bonamia infested oyster batches from North America to European waters. This has demonstrated the need for preventative measures, including:
- Monitoring oyster populations for health.
- Establishing zoning systems to limit the spread of parasites.
- Using appropriate management practices when transferring or introducing potential species for aquaculture.
Current shellfish regulations aim to address the latter issue by limiting the origin of imports to countries where no outbreak of disease occurs, according to the list of notifiable pathogens specified by the OIE International Aquatic Animal Health Code.
Within European waters, the implementation of EU directives prompted member states to develop a zoning system regarding for flat oysters to limit the spread of diseases.
Moreover, implementation of the FAO Code of Conduct for Responsible Fisheries (Article 9 – Aquaculture development), the ICES Code of Practices for Introduction and Transfer of Marine Organisms and recommendations for sustainable aquaculture from the Convention of Biological Diversity are of particular interest for this species. Implementation should include a precautionary approach to using genetic engineering strains, such as the development of a future disease-tolerant strain.
Further developments should take into account the fact that a genetic differentiation of the flat oyster populations along the European coastline still exists, in spite of numerous oyster transfers carried out over the last century. However, the population bottleneck and the small effective number of breeders available are expected to lead to increasing inbreeding and/or possible gene introgression into wild depleted populations. These facts are critically important for the future management of both cultured and wild populations of O. edulis.
June 2010




